Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation
- PMID: 20457835
- PMCID: PMC2889633
- DOI: 10.1152/ajpcell.00508.2009
Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation
Abstract
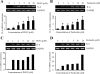
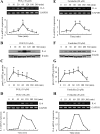
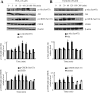
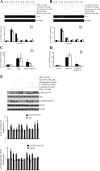
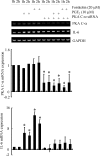
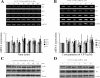
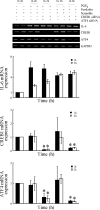
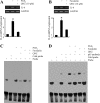
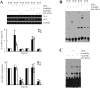
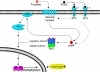
Elevated levels of prostaglandin (PG)E(2) and interleukin (IL)-6 have been reported in the cartilage and synovial fluid from patients with arthritic disorders. PGE(2) regulates IL-6 production in numerous different cells including macrophages and synovial fibroblasts. Although PGE(2) stimulates IL-6 expression in human chondrocytes, the underlying signaling pathway of this process has yet to be delineated. Here, we investigate the mechanism of IL-6 induction in human T/C-28a2 chondrocytes treated with exogenously added PGE(2). PGE(2) induces IL-6 mRNA and protein expression via a cAMP-dependent pathway, reaching maximal levels after 60 min of stimulation before declining to baseline levels at 6 h. Forskolin, an adenylyl cyclase activator, also stimulates IL-6 expression in human chondrocytes in a dose- and time-dependent fashion. Inhibition of downstream effectors of cAMP activity such as protein kinase A (PKA) or phosphatidylinositol 3 kinase (PI3K) blocks PGE(2)- and forskolin-induced IL-6 upregulation. Simultaneous inhibition of PKA and PI3K reduces IL-6 expression in stimulated chondrocytes well below the basal levels of untreated cells. Gel shift, supershift, and chromatin immunoprecipitation assays reveal the activation and binding of the nuclear factor (NF)-kappaB p65 subunit to the IL-6 promoter, which is markedly suppressed by selective PI3K or PKA pharmacological inhibitors. p65 knockdown completely abrogates IL-6 mRNA synthesis in PGE(2)- and forskolin-primed chondrocytes. Cumulatively, our data show that PGE(2) and forskolin induce IL-6 expression in human chondrocytes via cAMP/PKA and PI3K-dependent pathways, which in turn regulate the activation and binding of p65 to the IL-6 promoter.
Figures

Similar articles
-
Shear-induced interleukin-6 synthesis in chondrocytes: roles of E prostanoid (EP) 2 and EP3 in cAMP/protein kinase A- and PI3-K/Akt-dependent NF-kappaB activation.J Biol Chem. 2010 Aug 6;285(32):24793-804. doi: 10.1074/jbc.M110.110320. Epub 2010 Jun 1. J Biol Chem. 2010. PMID: 20516073 Free PMC article.
-
Interleukin-6 synthesis in human chondrocytes is regulated via the antagonistic actions of prostaglandin (PG)E2 and 15-deoxy-Δ(12,14)-PGJ2.PLoS One. 2011;6(11):e27630. doi: 10.1371/journal.pone.0027630. Epub 2011 Nov 11. PLoS One. 2011. Retraction in: PLoS One. 2024 Jul 30;19(7):e0306507. doi: 10.1371/journal.pone.0306507. PMID: 22096605 Free PMC article. Retracted.
-
Coordinate regulation of forskolin-induced cellular proliferation in macrophages by protein kinase A/cAMP-response element-binding protein (CREB) and Epac1-Rap1 signaling: effects of silencing CREB gene expression on Akt activation.J Biol Chem. 2005 Nov 18;280(46):38276-89. doi: 10.1074/jbc.M507332200. Epub 2005 Sep 19. J Biol Chem. 2005. PMID: 16172130
-
Phosphatidylinositol 3-kinase inhibitors: promising drug candidates for cancer therapy.Cancer Sci. 2008 Sep;99(9):1734-40. doi: 10.1111/j.1349-7006.2008.00891.x. Epub 2008 Jul 4. Cancer Sci. 2008. PMID: 18616528 Free PMC article. Review.
-
Cyclic AMP: a selective modulator of NF-κB action.Cell Mol Life Sci. 2011 Dec;68(23):3823-41. doi: 10.1007/s00018-011-0757-8. Epub 2011 Jul 9. Cell Mol Life Sci. 2011. PMID: 21744067 Free PMC article. Review.
Cited by
-
Influence of low-dose aspirin, resistance exercise, and sex on human skeletal muscle PGE2 /COX pathway activity.Physiol Rep. 2021 Mar;9(5):e14790. doi: 10.14814/phy2.14790. Physiol Rep. 2021. PMID: 33661544 Free PMC article.
-
Notch4-dependent antagonism of canonical TGF-β1 signaling defines unique temporal fluctuations of SMAD3 activity in sheared proximal tubular epithelial cells.Am J Physiol Renal Physiol. 2013 Jul 1;305(1):F123-33. doi: 10.1152/ajprenal.00594.2012. Epub 2013 Apr 10. Am J Physiol Renal Physiol. 2013. PMID: 23576639 Free PMC article.
-
Elevated immune-inflammatory signaling in mood disorders: a new therapeutic target?Expert Rev Neurother. 2012 Sep;12(9):1143-61. doi: 10.1586/ern.12.98. Expert Rev Neurother. 2012. PMID: 23039393 Free PMC article. Review.
-
Shear-induced interleukin-6 synthesis in chondrocytes: roles of E prostanoid (EP) 2 and EP3 in cAMP/protein kinase A- and PI3-K/Akt-dependent NF-kappaB activation.J Biol Chem. 2010 Aug 6;285(32):24793-804. doi: 10.1074/jbc.M110.110320. Epub 2010 Jun 1. J Biol Chem. 2010. PMID: 20516073 Free PMC article.
-
Stress-related hormone norepinephrine induces interleukin-6 expression in GES-1 cells.Braz J Med Biol Res. 2014 Feb;47(2):101-9. doi: 10.1590/1414-431X20133346. Epub 2014 Jan 17. Braz J Med Biol Res. 2014. PMID: 24519125 Free PMC article.
References
-
- Abulencia JP, Gaspard R, Healy ZR, Gaarde WA, Quackenbush J, Konstantopoulos K. Shear-induced cyclooxygenase-2 via a JNK2/c-Jun-dependent pathway regulates prostaglandin receptor expression in chondrocytic cells. J Biol Chem 278: 28388–28394, 2003 - PubMed
-
- Aoyama T, Liang B, Okamoto T, Matsusaki T, Nishijo K, Ishibe T, Yasura K, Nagayama S, Nakayama T, Nakamura T, Toguchida J. PGE2 signal through EP2 promotes the growth of articular chondrocytes. J Bone Miner Res 20: 377–389, 2005 - PubMed
-
- Attur M, Al-Mussawir HE, Patel J, Kitay A, Dave M, Palmer G, Pillinger MH, Abramson SB. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: evidence for signaling via the EP4 receptor. J Immunol 181: 5082–5088, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

