Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses
- PMID: 20451412
- PMCID: PMC2929482
- DOI: 10.1016/j.immuni.2010.04.011
Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses
Abstract
Dendritic cells (DCs) in mucosal surfaces are early targets for human immunodeficiency virus-1 (HIV-1). DCs mount rapid and robust immune responses upon pathogen encounter. However, immune response in the early events of HIV-1 transmission appears limited, suggesting that HIV-1 evade early immune control by DCs. We report that HIV-1 induces a rapid shutdown of autophagy and immunoamphisomes in DCs. HIV-1 envelope activated the mammalian target of rapamycin pathway in DCs, leading to autophagy exhaustion. HIV-1-induced inhibition of autophagy in DC increased cell-associated HIV-1 and transfer of HIV-1 infection to CD4(+) T cells. HIV-1-mediated downregulation of autophagy in DCs impaired innate and adaptive immune responses. Immunoamphisomes in DCs engulf incoming pathogens and appear to amplify pathogen degradation as well as Toll-like receptor responses and antigen presentation. The findings that HIV-1 downregulates autophagy and impedes immune functions of DCs represent a pathogenesis mechanism that can be pharmacologically countered with therapeutic and prophylactic implications.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
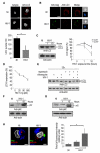

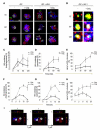
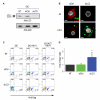
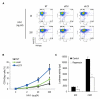
Comment in
-
Special delivery for MHC II via autophagy.Immunity. 2010 May 28;32(5):587-90. doi: 10.1016/j.immuni.2010.04.015. Immunity. 2010. PMID: 20510868
Similar articles
-
Immunoamphisomes in dendritic cells amplify TLR signaling and enhance exogenous antigen presentation on MHC-II.Autophagy. 2010 Aug;6(6):816-8. doi: 10.4161/auto.6.6.12623. Epub 2010 Aug 9. Autophagy. 2010. PMID: 20595805
-
Roles of autophagy in HIV infection.Immunol Cell Biol. 2015 Jan;93(1):11-7. doi: 10.1038/icb.2014.88. Epub 2014 Nov 11. Immunol Cell Biol. 2015. PMID: 25385065 Review.
-
Synthetic Abortive HIV-1 RNAs Induce Potent Antiviral Immunity.Front Immunol. 2020 Jan 23;11:8. doi: 10.3389/fimmu.2020.00008. eCollection 2020. Front Immunol. 2020. PMID: 32038656 Free PMC article.
-
HIV Exploits Antiviral Host Innate GCN2-ATF4 Signaling for Establishing Viral Replication Early in Infection.mBio. 2017 May 2;8(3):e01518-16. doi: 10.1128/mBio.01518-16. mBio. 2017. PMID: 28465428 Free PMC article.
-
HMGB1, an alarmin promoting HIV dissemination and latency in dendritic cells.Cell Death Differ. 2012 Jan;19(1):96-106. doi: 10.1038/cdd.2011.134. Epub 2011 Oct 28. Cell Death Differ. 2012. PMID: 22033335 Free PMC article. Review.
Cited by
-
DRAM triggers lysosomal membrane permeabilization and cell death in CD4(+) T cells infected with HIV.PLoS Pathog. 2013;9(5):e1003328. doi: 10.1371/journal.ppat.1003328. Epub 2013 May 2. PLoS Pathog. 2013. PMID: 23658518 Free PMC article. Clinical Trial.
-
Role of Autophagy in HIV Pathogenesis and Drug Abuse.Mol Neurobiol. 2017 Oct;54(8):5855-5867. doi: 10.1007/s12035-016-0118-6. Epub 2016 Sep 22. Mol Neurobiol. 2017. PMID: 27660273 Free PMC article. Review.
-
When autophagy meets viruses: a double-edged sword with functions in defense and offense.Semin Immunopathol. 2010 Dec;32(4):323-41. doi: 10.1007/s00281-010-0226-8. Epub 2010 Sep 25. Semin Immunopathol. 2010. PMID: 20865416 Free PMC article. Review.
-
Clearance of HIV-1 or SIV reservoirs by promotion of apoptosis and inhibition of autophagy: Targeting intracellular molecules in cure-directed strategies.J Leukoc Biol. 2022 Nov;112(5):1245-1259. doi: 10.1002/JLB.4MR0222-606. Epub 2022 Mar 31. J Leukoc Biol. 2022. PMID: 35362118 Free PMC article. Review.
-
How Viral and Intracellular Bacterial Pathogens Reprogram the Metabolism of Host Cells to Allow Their Intracellular Replication.Front Cell Infect Microbiol. 2019 Mar 4;9:42. doi: 10.3389/fcimb.2019.00042. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30886834 Free PMC article. Review.
References
-
- Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. - PubMed
-
- Buseyne F, Le Gall S, Boccaccio C, Abastado JP, Lifson JD, Arthur LO, Rivière Y, Heard JM, Schwartz O. MHC-I-restricted presentation of HIV-1 virion antigens without viral replication. Nat. Med. 2001;7:344–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

