Altered redox state of monocytes from cryopyrin-associated periodic syndromes causes accelerated IL-1beta secretion
- PMID: 20445104
- PMCID: PMC2906851
- DOI: 10.1073/pnas.1000779107
Altered redox state of monocytes from cryopyrin-associated periodic syndromes causes accelerated IL-1beta secretion
Abstract
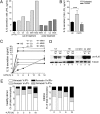
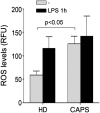
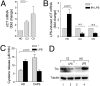
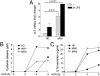
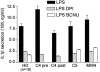
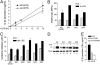
In healthy monocytes, Toll-like receptor (TLR) engagement induces production of reactive oxygen species (ROS), followed by an antioxidant response involved in IL-1beta processing and secretion. Markers of the antioxidant response include intracellular thioredoxin and extracellular release of reduced cysteine. Cryopyrin-associated periodic syndromes (CAPS) are autoinflammatory diseases in which Nod-like receptor family pyrin domain-containing 3 (NLRP3) gene mutations lead to increased IL-1beta secretion. We show in a large cohort of patients that IL-1beta secretion by CAPS monocytes is much faster than that by healthy monocytes. This accelerated kinetics is caused by alterations in the basal redox state, as well as in the redox response to TLR triggering displayed by CAPS monocytes. Indeed, unstimulated CAPS monocytes are under a mild oxidative stress, with elevated levels of both ROS and antioxidants. The redox response to LPS is quickened, with early generation of the reducing conditions favoring IL-1beta processing and secretion, and then rapidly exhausted. Therefore, secretion of IL-1beta is accelerated, but reaches a plateau much earlier than in healthy controls. Pharmacologic inhibition of the redox response hinders IL-1beta release, confirming the functional link between redox impairment and altered kinetics of secretion. Monocytes from patients with juvenile idiopathic arthritis display normal kinetics of redox response and IL-1beta secretion, excluding a role of chronic inflammation in the alterations observed in CAPS. We conclude that preexisting redox alterations distinct from CAPS monocytes anticipate the pathogen-associated molecular pattern molecule-induced generation of the reducing environment favorable to inflammasome activation and IL-1beta secretion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Increased NLRP3-dependent interleukin 1β secretion in patients with familial Mediterranean fever: correlation with MEFV genotype.Ann Rheum Dis. 2014 Feb;73(2):462-9. doi: 10.1136/annrheumdis-2012-202774. Epub 2013 Mar 16. Ann Rheum Dis. 2014. PMID: 23505242
-
Progressive waves of IL-1β release by primary human monocytes via sequential activation of vesicular and gasdermin D-mediated secretory pathways.Cell Death Dis. 2018 Oct 23;9(11):1088. doi: 10.1038/s41419-018-1121-9. Cell Death Dis. 2018. PMID: 30352992 Free PMC article.
-
Cell stress increases ATP release in NLRP3 inflammasome-mediated autoinflammatory diseases, resulting in cytokine imbalance.Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):2835-40. doi: 10.1073/pnas.1424741112. Epub 2015 Feb 17. Proc Natl Acad Sci U S A. 2015. PMID: 25730877 Free PMC article. Clinical Trial.
-
Redox stress unbalances the inflammatory cytokine network: role in autoinflammatory patients and healthy subjects.J Leukoc Biol. 2016 Jan;99(1):79-86. doi: 10.1189/jlb.3MR0415-159R. Epub 2015 Jul 21. J Leukoc Biol. 2016. PMID: 26199031 Free PMC article. Review.
-
Anakinra for cryopyrin-associated periodic syndrome.Expert Rev Clin Immunol. 2014 Jan;10(1):7-18. doi: 10.1586/1744666X.2014.861325. Epub 2013 Nov 21. Expert Rev Clin Immunol. 2014. PMID: 24308832 Review.
Cited by
-
A Knock-In Mouse Model of Cryopyrin-Associated Periodic Syndromes.Methods Mol Biol. 2023;2696:281-297. doi: 10.1007/978-1-0716-3350-2_19. Methods Mol Biol. 2023. PMID: 37578730 Review.
-
Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients.PLoS One. 2011;6(5):e20014. doi: 10.1371/journal.pone.0020014. Epub 2011 May 26. PLoS One. 2011. PMID: 21637346 Free PMC article.
-
Evolution, role in inflammation, and redox control of leaderless secretory proteins.J Biol Chem. 2020 May 29;295(22):7799-7811. doi: 10.1074/jbc.REV119.008907. Epub 2020 Apr 24. J Biol Chem. 2020. PMID: 32332096 Free PMC article. Review.
-
The NLRP3 and Pyrin Inflammasomes: Implications in the Pathophysiology of Autoinflammatory Diseases.Front Immunol. 2017 Jan 27;8:43. doi: 10.3389/fimmu.2017.00043. eCollection 2017. Front Immunol. 2017. PMID: 28191008 Free PMC article. Review.
-
Current status of understanding the pathogenesis and management of patients with NOMID/CINCA.Curr Rheumatol Rep. 2011 Apr;13(2):123-31. doi: 10.1007/s11926-011-0165-y. Curr Rheumatol Rep. 2011. PMID: 21538043 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

