Metal-assisted channel stabilization: disposition of a single histidine on the N-terminus of alamethicin yields channels with extraordinarily long lifetimes
- PMID: 20441743
- PMCID: PMC2862188
- DOI: 10.1016/j.bpj.2010.01.028
Metal-assisted channel stabilization: disposition of a single histidine on the N-terminus of alamethicin yields channels with extraordinarily long lifetimes
Abstract
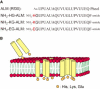
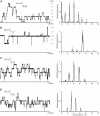
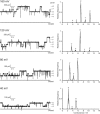
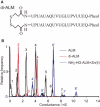
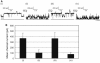
Alamethicin, a member of the peptaibol family of antibiotics, is a typical channel-forming peptide with a helical structure. The self-assembly of the peptide in the membranes yields voltage-dependent channels. In this study, three alamethicin analogs possessing a charged residue (His, Lys, or Glu) on their N-termini were designed with the expectation of stabilizing the transmembrane structure. A slight elongation of channel lifetime was observed for the Lys and Glu analogs. On the other hand, extensive stabilization of certain channel open states was observed for the His analog. This stabilization was predominantly observed in the presence of metal ions such as Zn(2+), suggesting that metal coordination with His facilitates the formation of a supramolecular assembly in the membranes. Channel stability was greatly diminished by acetylation of the N-terminal amino group, indicating that the N-terminal amino group also plays an important role in metal coordination.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Ion channel stabilization of synthetic alamethicin analogs by rings of inter-helix H-bonds.Biophys J. 1996 Apr;70(4):1669-75. doi: 10.1016/S0006-3495(96)79729-1. Biophys J. 1996. PMID: 8785325 Free PMC article.
-
Engineering stabilized ion channels: covalent dimers of alamethicin.Biochemistry. 1996 May 21;35(20):6225-32. doi: 10.1021/bi9529216. Biochemistry. 1996. PMID: 8639562
-
Conformational study of a synthetic analogue of alamethicin. Influence of the conformation on ion-channel lifetimes.Int J Pept Protein Res. 1995 Feb;45(2):164-72. doi: 10.1111/j.1399-3011.1995.tb01036.x. Int J Pept Protein Res. 1995. PMID: 7540163
-
Voltage-dependent pore formation and antimicrobial activity by alamethicin and analogues.J Membr Biol. 2001 Nov 1;184(1):1-12. doi: 10.1007/s00232-001-0077-2. J Membr Biol. 2001. PMID: 11687873 Review. No abstract available.
-
Model ion channels: gramicidin and alamethicin.J Membr Biol. 1992 Aug;129(2):109-36. doi: 10.1007/BF00219508. J Membr Biol. 1992. PMID: 1279177 Review.
Cited by
-
Epithelial barrier resistance is increased by the divalent cation zinc in cultured MDCKII epithelial monolayers.J Membr Biol. 2010 Oct;237(2-3):115-23. doi: 10.1007/s00232-010-9312-z. Epub 2010 Nov 6. J Membr Biol. 2010. PMID: 21057779
-
Systematic Design and Validation of Ion Channel Stabilization of Amphipathic α-Helical Peptides Incorporating Tryptophan Residues.ACS Omega. 2020 Dec 29;6(1):723-732. doi: 10.1021/acsomega.0c05254. eCollection 2021 Jan 12. ACS Omega. 2020. PMID: 33553860 Free PMC article.
References
-
- Woolley G.A., Lougheed T. Modeling ion channel regulation. Curr. Opin. Chem. Biol. 2003;7:710–714. - PubMed
-
- Sakai N., Mareda J., Matile S. Rigid-rod molecules in biomembrane models: from hydrogen-bonded chains to synthetic multifunctional pores. Acc. Chem. Res. 2005;38:79–87. - PubMed
-
- Bayley H., Jayasinghe L. Functional engineered channels and pores (Review) Mol. Membr. Biol. 2004;21:209–220. - PubMed
-
- Gokel G.W., Schlesinger P.H., Weber M.E. Functional, synthetic organic chemical models of cellular ion channels. Bioorg. Med. Chem. 2004;12:1291–1304. - PubMed
-
- Futaki S. Peptide ion channels: design and creation of function. Biopolymers. 1998;47:75–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

