Retinoids for treatment of retinal diseases
- PMID: 20435355
- PMCID: PMC2882531
- DOI: 10.1016/j.tips.2010.03.001
Retinoids for treatment of retinal diseases
Abstract
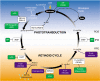
Knowledge about retinal photoreceptor signal transduction and the visual cycle required for normal eyesight has increased exponentially over the past decade. Substantial progress in human genetics has facilitated the identification of candidate genes and complex networks underlying inherited retinal diseases. Natural mutations in animal models that mimic human diseases have been characterized and advanced genetic manipulation can now be used to generate small mammalian models of human retinal diseases. Pharmacological repair of defective visual processes in animal models not only validates their involvement in vision, but also provides great promise for the development of improved therapies for millions who are progressing towards blindness or are almost completely robbed of their eyesight.
Figures



Similar articles
-
Structural approaches to understanding retinal proteins needed for vision.Curr Opin Cell Biol. 2014 Apr;27:32-43. doi: 10.1016/j.ceb.2013.11.001. Epub 2013 Nov 28. Curr Opin Cell Biol. 2014. PMID: 24680428 Free PMC article. Review.
-
Key enzymes of the retinoid (visual) cycle in vertebrate retina.Biochim Biophys Acta. 2012 Jan;1821(1):137-51. doi: 10.1016/j.bbalip.2011.03.005. Epub 2011 Apr 5. Biochim Biophys Acta. 2012. PMID: 21447403 Free PMC article. Review.
-
Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness.Proc Natl Acad Sci U S A. 2000 Jul 18;97(15):8623-8. doi: 10.1073/pnas.150236297. Proc Natl Acad Sci U S A. 2000. PMID: 10869443 Free PMC article.
-
Interpretations of fundus autofluorescence from studies of the bisretinoids of the retina.Invest Ophthalmol Vis Sci. 2010 Sep;51(9):4351-7. doi: 10.1167/iovs.10-5852. Invest Ophthalmol Vis Sci. 2010. PMID: 20805567 Free PMC article.
-
Improvement of visual performance with intravitreal administration of 9-cis-retinal in Rpe65-mutant dogs.Arch Ophthalmol. 2010 Nov;128(11):1442-8. doi: 10.1001/archophthalmol.2010.210. Epub 2010 Sep 13. Arch Ophthalmol. 2010. PMID: 20837787
Cited by
-
Chemistry of the retinoid (visual) cycle.Chem Rev. 2014 Jan 8;114(1):194-232. doi: 10.1021/cr400107q. Epub 2013 Jul 11. Chem Rev. 2014. PMID: 23905688 Free PMC article. Review. No abstract available.
-
The emerging roles of melanopsin in behavioral adaptation to light.Trends Mol Med. 2010 Oct;16(10):435-46. doi: 10.1016/j.molmed.2010.07.005. Epub 2010 Aug 31. Trends Mol Med. 2010. PMID: 20810319 Free PMC article. Review.
-
All-trans-retinal induces Bax activation via DNA damage to mediate retinal cell apoptosis.Exp Eye Res. 2014 Jun;123:27-36. doi: 10.1016/j.exer.2014.04.003. Epub 2014 Apr 12. Exp Eye Res. 2014. PMID: 24726920 Free PMC article.
-
Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal.J Biol Chem. 2013 May 24;288(21):15326-41. doi: 10.1074/jbc.M112.448712. Epub 2013 Apr 9. J Biol Chem. 2013. PMID: 23572532 Free PMC article.
-
Peptide Derivatives of Retinylamine Prevent Retinal Degeneration with Minimal Side Effects on Vision in Mice.Bioconjug Chem. 2021 Mar 17;32(3):572-583. doi: 10.1021/acs.bioconjchem.1c00043. Epub 2021 Mar 7. Bioconjug Chem. 2021. PMID: 33677964 Free PMC article.
References
-
- Thompson DA, Gal A. Vitamin A metabolism in the retinal pigment epithelium: genes, mutations, and diseases. Progress in retinal and eye research. 2003;22:683–703. - PubMed
-
- Jackson GR, et al. Impact of aging and age-related maculopathy on inactivation of the a-wave of the rod-mediated electroretinogram. Vision research. 2006;46:1422–1431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

