Phosphatidic acid mediates activation of mTORC1 through the ERK signaling pathway
- PMID: 20427710
- PMCID: PMC2928642
- DOI: 10.1152/ajpcell.00039.2010
Phosphatidic acid mediates activation of mTORC1 through the ERK signaling pathway
Abstract
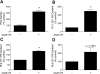
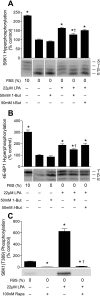
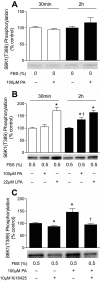
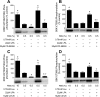
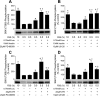
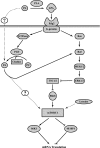
The mammalian target of rapamycin (mTOR) assembles into two distinct multiprotein complexes known as mTORC1 and mTORC2. Of the two complexes, mTORC1 acts to integrate a variety of positive and negative signals to downstream targets that regulate cell growth. The lipid second messenger, phosphatidic acid (PA), represents one positive input to mTORC1, and it is thought to act by binding directly to mTOR, thereby enhancing the protein kinase activity of mTORC1. Support for this model includes findings that PA binds directly to mTOR and addition of PA to the medium of cells in culture results in activation of mTORC1. In contrast, the results of the present study do not support a model in which PA activates mTORC1 through direct interaction with the protein kinase but, instead, show that the lipid promotes mTORC1 signaling through activation of the ERK pathway. Moreover, rather than acting directly on mTORC1, the results suggest that exogenous PA must be metabolized to lysophosphatidic acid (LPA), which subsequently activates the LPA receptor endothelial differentiation gene (EDG-2). Finally, in contrast to previous studies, the results of the present study demonstrate that leucine does not act through phospholipase D and PA to activate mTORC1 and, instead, show that the two mediators act through parallel upstream signaling pathways to activate mTORC1. Overall, the results demonstrate that leucine and PA signal through parallel pathways to activate mTORC1 and that PA mediates its effect through the ERK pathway, rather than through direct binding to mTOR.
Figures
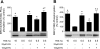
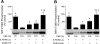
Similar articles
-
Phosphatidic acid activates mammalian target of rapamycin complex 1 (mTORC1) kinase by displacing FK506 binding protein 38 (FKBP38) and exerting an allosteric effect.J Biol Chem. 2011 Aug 26;286(34):29568-74. doi: 10.1074/jbc.M111.262816. Epub 2011 Jul 7. J Biol Chem. 2011. PMID: 21737445 Free PMC article.
-
Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin.Mol Cell Biol. 2009 Mar;29(6):1411-20. doi: 10.1128/MCB.00782-08. Epub 2008 Dec 29. Mol Cell Biol. 2009. PMID: 19114562 Free PMC article.
-
Amino acid regulation of TOR complex 1.Am J Physiol Endocrinol Metab. 2009 Apr;296(4):E592-602. doi: 10.1152/ajpendo.90645.2008. Epub 2008 Sep 2. Am J Physiol Endocrinol Metab. 2009. PMID: 18765678 Free PMC article. Review.
-
The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle.Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4741-6. doi: 10.1073/pnas.0600678103. Epub 2006 Mar 14. Proc Natl Acad Sci U S A. 2006. PMID: 16537399 Free PMC article.
-
Phosphatidic acid signaling to mTOR: signals for the survival of human cancer cells.Biochim Biophys Acta. 2009 Sep;1791(9):949-55. doi: 10.1016/j.bbalip.2009.02.009. Epub 2009 Mar 2. Biochim Biophys Acta. 2009. PMID: 19264150 Free PMC article. Review.
Cited by
-
The effects of phosphatidic acid supplementation on strength, body composition, muscular endurance, power, agility, and vertical jump in resistance trained men.J Int Soc Sports Nutr. 2016 Jun 2;13:24. doi: 10.1186/s12970-016-0135-x. eCollection 2016. J Int Soc Sports Nutr. 2016. PMID: 27274715 Free PMC article. Clinical Trial.
-
Ultralow Background Membrane Editors for Spatiotemporal Control of Phosphatidic Acid Metabolism and Signaling.ACS Cent Sci. 2024 Jan 30;10(3):543-554. doi: 10.1021/acscentsci.3c01105. eCollection 2024 Mar 27. ACS Cent Sci. 2024. PMID: 38559292 Free PMC article.
-
Signal transduction in inherited metabolic disorders: a model for a possible pathogenetic mechanism.J Inherit Metab Dis. 2015 Jul;38(4):729-40. doi: 10.1007/s10545-015-9820-1. Epub 2015 Mar 4. J Inherit Metab Dis. 2015. PMID: 25735935 Review.
-
Oleic acid stimulation of amino acid uptake in primary human trophoblast cells is mediated by phosphatidic acid and mTOR signaling.FASEB Bioadv. 2023 Nov 14;6(1):1-11. doi: 10.1096/fba.2023-00113. eCollection 2024 Jan. FASEB Bioadv. 2023. PMID: 38223199 Free PMC article.
-
Mechanisms mediating the effects of alcohol and HIV anti-retroviral agents on mTORC1, mTORC2 and protein synthesis in myocytes.World J Biol Chem. 2012 Jun 26;3(6):110-20. doi: 10.4331/wjbc.v3.i6.110. World J Biol Chem. 2012. PMID: 22905289 Free PMC article.
References
-
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulous GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

