Tandem fluorescence imaging of dynamic S-acylation and protein turnover
- PMID: 20421494
- PMCID: PMC2889305
- DOI: 10.1073/pnas.0912306107
Tandem fluorescence imaging of dynamic S-acylation and protein turnover
Abstract
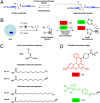
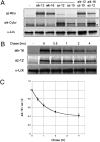
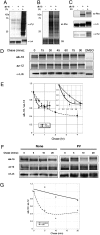
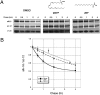
The functional significance and regulation of reversible S-acylation on diverse proteins remain unclear because of limited methods for efficient quantitative analysis of palmitate turnover. Here, we describe a tandem labeling and detection method to simultaneously monitor dynamic S-palmitoylation and protein turnover. By combining S-acylation and cotranslational fatty acid chemical reporters with orthogonal clickable fluorophores, dual pulse-chase analysis of Lck revealed accelerated palmitate cycling upon T-cell activation. Subsequent pharmacological perturbation of Lck palmitate turnover suggests yet uncharacterized serine hydrolases contribute to dynamic S-acylation in cells. In addition to dually fatty-acylated proteins, this tandem fluorescence imaging method can be generalized to other S-acylated proteins using azidohomoalanine as a methonine surrogate. The sensitivity and efficiency of this approach should facilitate the functional characterization of cellular factors and drugs that modulate protein S-acylation. Furthermore, diverse protein modifications could be analyzed with this tandem imaging method using other chemical reporters to investigate dynamic regulation of protein function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
DHHC2 is a protein S-acyltransferase for Lck.Mol Membr Biol. 2011 Oct-Nov;28(7-8):473-86. doi: 10.3109/09687688.2011.630682. Mol Membr Biol. 2011. PMID: 22034844
-
Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids.J Biol Chem. 2000 Jan 7;275(1):261-70. doi: 10.1074/jbc.275.1.261. J Biol Chem. 2000. PMID: 10617614
-
S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes.EMBO J. 1997 Aug 15;16(16):4983-98. doi: 10.1093/emboj/16.16.4983. EMBO J. 1997. PMID: 9305640 Free PMC article.
-
Protein S-palmitoylation in cellular differentiation.Biochem Soc Trans. 2017 Feb 8;45(1):275-285. doi: 10.1042/BST20160236. Biochem Soc Trans. 2017. PMID: 28202682 Free PMC article. Review.
-
The role of competing mechanisms on Lck regulation.Immunol Res. 2020 Oct;68(5):289-295. doi: 10.1007/s12026-020-09148-2. Epub 2020 Aug 14. Immunol Res. 2020. PMID: 32794043 Review.
Cited by
-
Emerging roles for protein S-palmitoylation in immunity from chemical proteomics.Curr Opin Chem Biol. 2013 Feb;17(1):27-33. doi: 10.1016/j.cbpa.2012.11.008. Epub 2013 Jan 14. Curr Opin Chem Biol. 2013. PMID: 23332315 Free PMC article. Review.
-
Nutrient-Dependent Changes of Protein Palmitoylation: Impact on Nuclear Enzymes and Regulation of Gene Expression.Int J Mol Sci. 2018 Nov 30;19(12):3820. doi: 10.3390/ijms19123820. Int J Mol Sci. 2018. PMID: 30513609 Free PMC article. Review.
-
Fat chance! Getting a grip on a slippery modification.ACS Chem Biol. 2013 Jan 18;8(1):46-57. doi: 10.1021/cb300607e. Epub 2012 Dec 18. ACS Chem Biol. 2013. PMID: 23215442 Free PMC article. Review.
-
Exploring protein lipidation with chemical biology.Chem Rev. 2011 Oct 12;111(10):6341-58. doi: 10.1021/cr2001977. Epub 2011 Sep 16. Chem Rev. 2011. PMID: 21919527 Free PMC article. Review. No abstract available.
-
A fluorescent probe for cysteine depalmitoylation reveals dynamic APT signaling.Nat Chem Biol. 2017 Feb;13(2):150-152. doi: 10.1038/nchembio.2262. Epub 2016 Dec 19. Nat Chem Biol. 2017. PMID: 27992880 Free PMC article.
References
-
- Resh MD. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat Chem Biol. 2006;2:584–590. - PubMed
-
- Linder ME, Deschenes RJ. Palmitoylation: Policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. - PubMed
-
- Rocks O, et al. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

