Adenine oxidation by pyrite-generated hydroxyl radicals
- PMID: 20420694
- PMCID: PMC2873965
- DOI: 10.1186/1467-4866-11-2
Adenine oxidation by pyrite-generated hydroxyl radicals
Abstract
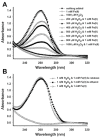
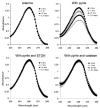
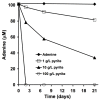
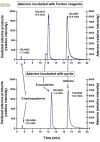
Cellular exposure to particulate matter with concomitant formation of reactive oxygen species (ROS) and oxidization of biomolecules may lead to negative health outcomes. Evaluating the particle-induced formation of ROS and the oxidation products from reaction of ROS with biomolecules is useful for gaining a mechanistic understanding of particle-induced oxidative stress. Aqueous suspensions of pyrite particles have been shown to form hydroxyl radicals and degrade nucleic acids. Reactions between pyrite-induced hydroxyl radicals and nucleic acid bases, however, remain to be determined. Here, we compared the oxidation of adenine by Fenton-generated (i.e., ferrous iron and hydrogen peroxide) hydroxyl radicals to adenine oxidation by hydroxyl radicals generated in pyrite aqueous suspensions. Results show that adenine oxidizes in the presence of pyrite (without the addition of hydrogen peroxide) and that the rate of oxidation is dependent on the pyrite loading. Adenine oxidation was prevented by addition of either catalase or ethanol to the pyrite/adenine suspensions, which implies that hydrogen peroxide and hydroxyl radicals are causing the adenine oxidation. The adenine oxidation products, 8-oxoadenine and 2-hydroxyadenine, were the same whether hydroxyl radicals were generated by Fenton or pyrite-initiated reactions. Although nucleic acid bases are unlikely to be directly exposed to pyrite particles, the formation of ROS in the vicinity of cells may lead to oxidative stress.
Figures
Similar articles
-
Pyrite-induced hydroxyl radical formation and its effect on nucleic acids.Geochem Trans. 2006 Apr 4;7:3. doi: 10.1186/1467-4866-7-3. Geochem Trans. 2006. PMID: 16759350 Free PMC article.
-
Novel visible light enhanced Pyrite-Fenton system toward ultrarapid oxidation of p-nitrophenol: Catalytic activity, characterization and mechanism.Chemosphere. 2019 Aug;228:232-240. doi: 10.1016/j.chemosphere.2019.04.103. Epub 2019 Apr 16. Chemosphere. 2019. PMID: 31035160
-
Mechanisms of Sb(III) oxidation by pyrite-induced hydroxyl radicals and hydrogen peroxide.Environ Sci Technol. 2015 Mar 17;49(6):3499-505. doi: 10.1021/es505584r. Epub 2015 Mar 4. Environ Sci Technol. 2015. PMID: 25714842
-
[Free oxygen radiacals and kidney diseases--part I].Med Pregl. 2000 Sep-Oct;53(9-10):463-74. Med Pregl. 2000. PMID: 11320727 Review. Croatian.
-
Hydroxyl radical generations form the physiologically relevant Fenton-like reactions.Free Radic Biol Med. 2023 Nov 1;208:510-515. doi: 10.1016/j.freeradbiomed.2023.09.013. Epub 2023 Sep 17. Free Radic Biol Med. 2023. PMID: 37717792 Review.
Cited by
-
Phenylalanine as a hydroxyl radical-specific probe in pyrite slurries.Geochem Trans. 2012 Feb 7;13:3. doi: 10.1186/1467-4866-13-3. Geochem Trans. 2012. PMID: 22313632 Free PMC article.
-
Quantifying Fenton reaction pathways driven by self-generated H2O2 on pyrite surfaces.Sci Rep. 2017 Mar 6;7:43703. doi: 10.1038/srep43703. Sci Rep. 2017. PMID: 28262831 Free PMC article.
-
Genetic Diversity as Consequence of a Microaerobic and Neutrophilic Lifestyle.PLoS Pathog. 2016 May 11;12(5):e1005626. doi: 10.1371/journal.ppat.1005626. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27166672 Free PMC article.
-
What makes a natural clay antibacterial?Environ Sci Technol. 2011 Apr 15;45(8):3768-73. doi: 10.1021/es1040688. Epub 2011 Mar 17. Environ Sci Technol. 2011. PMID: 21413758 Free PMC article.
-
Cobalamin Protection against Oxidative Stress in the Acidophilic Iron-oxidizing Bacterium Leptospirillum Group II CF-1.Front Microbiol. 2016 May 23;7:748. doi: 10.3389/fmicb.2016.00748. eCollection 2016. Front Microbiol. 2016. PMID: 27242761 Free PMC article.
References
-
- Borda M, Elsetinow A, Schoonen M, Strongin D. Pyrite-induced hydrogen peroxide formation as a driving force in the evolution of photosynthetic organisms on an early Earth. Astrobiology. 2001;1:283–288. - PubMed
-
- Cohn CA, Borda MJ, Schoonen MA. RNA decomposition by pyrite-induced radicals and possible role of lipids during the emergence of life. Earth and Planetary Science Letters. 2004;225:271–278.
-
- Pryor WA. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annual Review of Physiology. 1986;48:657–663. - PubMed
LinkOut - more resources
Full Text Sources

