Inhibiting the inhibitors: evaluating agents targeting cancer immunosuppression
- PMID: 20415597
- PMCID: PMC2882984
- DOI: 10.1517/14712598.2010.482207
Inhibiting the inhibitors: evaluating agents targeting cancer immunosuppression
Abstract
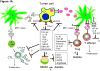
Importance of the field: Immunotherapy of cancer has not improved disease-free or overall patient survival. The lack of concordance between immunological and clinical responses in cancer immunotherapy trials is thought to result from the pervasive presence of tumor-driven immune suppression that allows tumor to escape and that has not been adequately targeted by current therapies.
Areas covered in this review: Because multiple mechanisms of tumor induced suppression have been identified and shown to contribute to tumor escape, the opportunity arises to interfere with these mechanisms. A range of known tumor-derived inhibitors can now be blocked or neutralized by biologic or metabolic agents. Used alone or in combination with each other or with conventional cancer therapies, these agents offer novel therapeutic strategies for the control of tumor escape.
What the reader will gain: This review deals with currently available inhibitors for counteracting tumor immune escape. The restoration of effective anti-tumor immunity in patients with cancer will require new approaches aiming at: i) protection of immune cells from adverse effects of myeloid-derived suppressor cells, regulatory T cells or inhibitory factors thus enhancing effector functions; and ii) prolonging survival of central memory T cells, thus ensuring long-term protection.
Take home message: Inhibitors of mechanisms responsible for tumor escape could restore anti-tumor immune responses in patients with cancer.
Conflict of interest statement
Declaration of interest
This paper has been sponsored by NIH Grants PO1-CA109688 to TLW and RO1-CA112643 to D. Shin (TLW co-investigator).
Figures

Similar articles
-
Prevailing over T cell exhaustion: New developments in the immunotherapy of pancreatic cancer.Cancer Lett. 2016 Oct 10;381(1):259-68. doi: 10.1016/j.canlet.2016.02.057. Epub 2016 Mar 8. Cancer Lett. 2016. PMID: 26968250 Review.
-
T-cell modulation by cyclophosphamide for tumour therapy.Immunology. 2018 May;154(1):62-68. doi: 10.1111/imm.12913. Epub 2018 Mar 9. Immunology. 2018. PMID: 29460448 Free PMC article. Review.
-
Tumor infiltrating regulatory T cells: tractable targets for immunotherapy.Int Rev Immunol. 2010 Oct;29(5):461-84. doi: 10.3109/08830185.2010.508854. Int Rev Immunol. 2010. PMID: 20839911 Review.
-
A Systematic Review of Immunotherapy in Urologic Cancer: Evolving Roles for Targeting of CTLA-4, PD-1/PD-L1, and HLA-G.Eur Urol. 2015 Aug;68(2):267-79. doi: 10.1016/j.eururo.2015.02.032. Epub 2015 Mar 29. Eur Urol. 2015. PMID: 25824720 Review.
-
Directing Traffic: How to Effectively Drive T Cells into Tumors.Cancer Discov. 2020 Feb;10(2):185-197. doi: 10.1158/2159-8290.CD-19-0790. Epub 2020 Jan 23. Cancer Discov. 2020. PMID: 31974169 Free PMC article. Review.
Cited by
-
Programmed death-1 checkpoint blockade in acute myeloid leukemia.Expert Opin Biol Ther. 2015;15(8):1191-203. doi: 10.1517/14712598.2015.1051028. Epub 2015 Jun 3. Expert Opin Biol Ther. 2015. PMID: 26036819 Free PMC article. Review.
-
Plasma membrane vesicles decorated with glycolipid-anchored antigens and adjuvants via protein transfer as an antigen delivery platform for inhibition of tumor growth.Biomaterials. 2016 Jan;74:231-44. doi: 10.1016/j.biomaterials.2015.09.031. Epub 2015 Sep 28. Biomaterials. 2016. PMID: 26461116 Free PMC article.
-
Modeling Macrophage Polarization and Its Effect on Cancer Treatment Success.Open J Immunol. 2018 Jun;8(2):36-80. doi: 10.4236/oji.2018.82004. Epub 2018 Jun 29. Open J Immunol. 2018. PMID: 35847834 Free PMC article.
-
Prognostic impact of expression of Bcl-2 and Bax genes in circulating immune cells derived from patients with head and neck carcinoma.Neoplasia. 2013 Mar;15(3):305-14. doi: 10.1593/neo.121528. Neoplasia. 2013. PMID: 23479508 Free PMC article.
-
Induced regulatory T cells in inhibitory microenvironments created by cancer.Expert Opin Biol Ther. 2014 Oct;14(10):1411-25. doi: 10.1517/14712598.2014.927432. Epub 2014 Jun 17. Expert Opin Biol Ther. 2014. PMID: 24934899 Free PMC article. Review.
References
-
- Sahin U, Tureci O, Pfreundschuh M. Serological identification of human tumor antigens. Curr Opin Immunol. 1997;9:709–16. - PubMed
-
- Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. - PubMed
-
- Whiteside TL. Immune effector cells in the tumor microenvironment: their role in regulation of tumor progression. In: Yefenof E, editor. Innate and Adaptive Immunity Responses in the Tumor Microenvironment. Springer Science; The Netherlands: 2008. pp. 1–34.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
