Utility of a commercial nonstructural protein 1 antigen capture kit as a dengue virus diagnostic tool
- PMID: 20410325
- PMCID: PMC2884419
- DOI: 10.1128/CVI.00041-10
Utility of a commercial nonstructural protein 1 antigen capture kit as a dengue virus diagnostic tool
Abstract
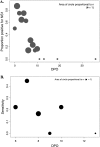
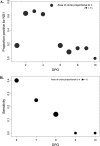
Annually, over 2.5 billion people are at risk for infection with dengue virus (DENV), while between 50 and 100 million people contract the infection. There is an urgent need for alternative diagnostic tools that can detect DENV during acute infection. Recent studies have shown that DENV nonstructural protein 1 (NS1) is detectable in the blood as early as the onset of symptoms and persists well into the convalescent phase of the infection. We evaluated the utility of the Bio-Rad Platelia DENV NS1 antigen capture kit in combination with real-time reverse transcriptase PCR (RT-PCR) and an IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA) for refining a new algorithm for the diagnosis of acute- or convalescent-phase DENV infection with a single clinical sample. We tested the Bio-Rad kit with three panels of sera. These panels were designed to evaluate the sensitivities of the NS1 kit for (i) early-convalescent-phase samples, (ii) acute-phase samples with false-negative PCR results, and (iii) IgM-negative convalescent-phase samples from patients with confirmed secondary DENV infections. Results show that NS1 can be detected in 22% of serum samples collected more than 10 days after the onset of illness and in 22% of samples that did not elicit an IgM response. Additionally, NS1 was detected in 37% of the tested acute-phase samples with false-negative PCR results, suggesting that NS1 detection may be valuable in increasing the sensitivity of current acute-phase diagnostics. These results will improve diagnosis with a single acute-phase or early-convalescent-phase sample for disease surveillance and clinical diagnosis.
Figures

Similar articles
-
Evaluation of a commercial SD dengue virus NS1 antigen capture enzyme-linked immunosorbent assay kit for early diagnosis of dengue virus infection.J Clin Microbiol. 2010 Aug;48(8):2793-7. doi: 10.1128/JCM.02142-09. Epub 2010 Jun 23. J Clin Microbiol. 2010. PMID: 20573879 Free PMC article.
-
Assessment of diagnostic and analytic performance of the SD Bioline Dengue Duo test for dengue virus (DENV) infections in an endemic area (Savannakhet province, Lao People's Democratic Republic).PLoS One. 2020 Mar 17;15(3):e0230337. doi: 10.1371/journal.pone.0230337. eCollection 2020. PLoS One. 2020. PMID: 32182271 Free PMC article.
-
Evaluation of two new commercial tests for the diagnosis of acute dengue virus infection using NS1 antigen detection in human serum.PLoS Negl Trop Dis. 2008 Aug 20;2(8):e280. doi: 10.1371/journal.pntd.0000280. PLoS Negl Trop Dis. 2008. PMID: 18714359 Free PMC article.
-
False-Positive Nonstructural Protein 1 Antigen in a Patient with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Case Report with Literature Review.Am J Case Rep. 2021 Apr 9;22:e928865. doi: 10.12659/AJCR.928865. Am J Case Rep. 2021. PMID: 33835996 Free PMC article. Review.
-
Dengue Detection: Advances in Diagnostic Tools from Conventional Technology to Point of Care.Biosensors (Basel). 2021 Jun 23;11(7):206. doi: 10.3390/bios11070206. Biosensors (Basel). 2021. PMID: 34201849 Free PMC article. Review.
Cited by
-
Development and validation of an ELISA kit (YF MAC-HD) to detect IgM to yellow fever virus.J Virol Methods. 2015 Dec 1;225:41-8. doi: 10.1016/j.jviromet.2015.08.025. Epub 2015 Sep 3. J Virol Methods. 2015. PMID: 26342907 Free PMC article.
-
Utility of the tourniquet test and the white blood cell count to differentiate dengue among acute febrile illnesses in the emergency room.PLoS Negl Trop Dis. 2011 Dec;5(12):e1400. doi: 10.1371/journal.pntd.0001400. Epub 2011 Dec 6. PLoS Negl Trop Dis. 2011. PMID: 22163057 Free PMC article.
-
Corticosteroids in the treatment of dengue shock syndrome.Infect Drug Resist. 2014 May 22;7:137-43. doi: 10.2147/IDR.S55380. eCollection 2014. Infect Drug Resist. 2014. PMID: 24899817 Free PMC article. Review.
-
High performance dengue virus antigen-based serotyping-NS1-ELISA (plus): A simple alternative approach to identify dengue virus serotypes in acute dengue specimens.PLoS Negl Trop Dis. 2021 Feb 26;15(2):e0009065. doi: 10.1371/journal.pntd.0009065. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33635874 Free PMC article.
-
Evaluation of NS1 Antigen Detection for Early Diagnosis of Dengue in a Tertiary Hospital in Southern India.J Clin Diagn Res. 2016 Apr;10(4):DC01-4. doi: 10.7860/JCDR/2016/15758.7562. Epub 2016 Apr 1. J Clin Diagn Res. 2016. PMID: 27190798 Free PMC article.
References
-
- Blacksell, S. D., M. P. Mammen, Jr., S. Thongpaseuth, R. V. Gibbons, R. G. Jarman, K. Jenjaroen, A. Nisalak, R. Phetsouvanh, P. N. Newton, and N. P. Day. 2008. Evaluation of the Panbio dengue virus nonstructural 1 antigen detection and immunoglobulin M antibody enzyme-linked immunosorbent assays for the diagnosis of acute dengue infections in Laos. Diagn. Microbiol. Infect. Dis. 60:43-49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

