Androgens and spermatogenesis: lessons from transgenic mouse models
- PMID: 20403868
- PMCID: PMC2871915
- DOI: 10.1098/rstb.2009.0117
Androgens and spermatogenesis: lessons from transgenic mouse models
Abstract
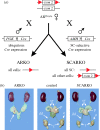
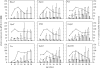
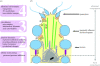
Transgenic mouse models have contributed considerably to our understanding of the cellular and molecular mechanisms by which androgens control spermatogenesis. Cell-selective ablation of the androgen receptor (AR) in Sertoli cells (SC) results in a complete block in meiosis and unambiguously identifies the SC as the main cellular mediator of the effects of androgens on spermatogenesis. This conclusion is corroborated by similar knockouts in other potential testicular target cells. Mutations resulting in diminished expression of the AR or in alleles with increased length of the CAG repeat mimick specific human forms of disturbed fertility that are not accompanied by defects in male sexual development. Transcriptional profiling studies in mice with cell-selective and general knockouts of the AR, searching for androgen-regulated genes relevant to the control of spermatogenesis, have identified many candidate target genes. However, with the exception of Rhox5, the identified subsets of genes show little overlap. Genes related to tubular restructuring, cell junction dynamics, the cytoskeleton, solute transportation and vitamin A metabolism are prominently present. Further research will be needed to decide which of these genes are physiologically relevant and to identify genes that can be used as diagnostic tools or targets to modulate the effects of androgens in spermatogenesis.
Figures
Similar articles
-
The role of androgens in sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor.Endocrinology. 2005 Jun;146(6):2674-83. doi: 10.1210/en.2004-1630. Epub 2005 Mar 10. Endocrinology. 2005. PMID: 15761038
-
Early effects of Sertoli cell-selective androgen receptor ablation on testicular gene expression.Int J Androl. 2010 Jun 1;33(3):507-17. doi: 10.1111/j.1365-2605.2009.00964.x. Epub 2009 Apr 12. Int J Androl. 2010. PMID: 19392831
-
Selective ablation of the androgen receptor in mouse sertoli cells affects sertoli cell maturation, barrier formation and cytoskeletal development.PLoS One. 2010 Nov 30;5(11):e14168. doi: 10.1371/journal.pone.0014168. PLoS One. 2010. PMID: 21152390 Free PMC article.
-
Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice.Endocr Rev. 2009 Apr;30(2):119-32. doi: 10.1210/er.2008-0025. Epub 2009 Jan 27. Endocr Rev. 2009. PMID: 19176467 Free PMC article. Review.
-
The regulation of spermatogenesis by androgens.Semin Cell Dev Biol. 2014 Jun;30:2-13. doi: 10.1016/j.semcdb.2014.02.012. Epub 2014 Mar 2. Semin Cell Dev Biol. 2014. PMID: 24598768 Free PMC article. Review.
Cited by
-
Unveiling the critical role of androgen receptor signaling in avian sexual development.Nat Commun. 2024 Oct 17;15(1):8970. doi: 10.1038/s41467-024-52989-w. Nat Commun. 2024. PMID: 39419984 Free PMC article.
-
17β-estradiol signaling and regulation of Sertoli cell function.Spermatogenesis. 2011 Oct;1(4):318-324. doi: 10.4161/spmg.1.4.18903. Epub 2011 Oct 1. Spermatogenesis. 2011. PMID: 22332115 Free PMC article.
-
Updates in Sertoli Cell-Mediated Signaling During Spermatogenesis and Advances in Restoring Sertoli Cell Function.Front Endocrinol (Lausanne). 2022 May 4;13:897196. doi: 10.3389/fendo.2022.897196. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35600584 Free PMC article. Review.
-
TAp73 is required for spermatogenesis and the maintenance of male fertility.Proc Natl Acad Sci U S A. 2014 Feb 4;111(5):1843-8. doi: 10.1073/pnas.1323416111. Epub 2014 Jan 21. Proc Natl Acad Sci U S A. 2014. PMID: 24449892 Free PMC article.
-
Concordant Androgen-Regulated Expression of Divergent Rhox5 Promoters in Sertoli Cells.Endocrinology. 2022 Jan 1;163(1):bqab237. doi: 10.1210/endocr/bqab237. Endocrinology. 2022. PMID: 34902009 Free PMC article.
References
-
- Abel M. H., Wootton A. N., Wilkins V., Huhtaniemi I., Knight P. G., Charlton H. M.2000The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 141, 1795–1803 (doi:10.1210/en.141.5.1795) - DOI - PubMed
-
- Abel M. H., Baker P. J., Charlton H. M., Monteiro A., Verhoeven G., De Gendt K., Guillou F., O'Shaughnessy P. J.2008Spermatogenesis and Sertoli cell activity in mice lacking Sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology 149, 3279–3285 (doi:10.1210/en.2008-0086) - DOI - PMC - PubMed
-
- Allan C. M., et al. 2001A novel transgenic model to characterize the specific effects of follicle-stimulating hormone on gonadal physiology in the absence of luteinizing hormone actions. Endocrinology 142, 2213–2220 (doi:10.1210/en.142.6.2213) - DOI - PubMed
-
- Au C. L., Irby D. C., Robertson D. M., deKretser D. M.1986Effects of testosterone on testicular inhibin and fluid production in intact and hypophysectomized adult-rats. J. Reprod. Fertil. 76, 257–266 - PubMed
-
- Barbulescu K., Geserick C., Schuttke I., Schleuning W. D., Haendler B.2001New androgen response elements in the murine Pem promoter mediate selective transactivation. Mol. Endocrinol. 15, 1803–1816 (doi:10.1210/me.15.10.1803) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

