Caveolin-1 modulates HIV-1 envelope-induced bystander apoptosis through gp41
- PMID: 20392844
- PMCID: PMC2903242
- DOI: 10.1128/JVI.02722-09
Caveolin-1 modulates HIV-1 envelope-induced bystander apoptosis through gp41
Abstract
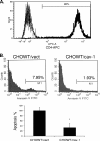
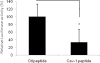
Human immunodeficiency virus (HIV) envelope (Env)-mediated bystander apoptosis is known to cause the progressive, severe, and irreversible loss of CD4(+) T cells in HIV-1-infected patients. Env-induced bystander apoptosis has been shown to be gp41 dependent and related to the membrane hemifusion between envelope-expressing cells and target cells. Caveolin-1 (Cav-1), the scaffold protein of specific membrane lipid rafts called caveolae, has been reported to interact with gp41. However, the underlying pathological or physiological meaning of this robust interaction remains unclear. In this report, we examine the interaction of cellular Cav-1 and HIV gp41 within the lipid rafts and show that Cav-1 modulates Env-induced bystander apoptosis through interactions with gp41 in SupT1 cells and CD4(+) T lymphocytes isolated from human peripheral blood. Cav-1 significantly suppressed Env-induced membrane hemifusion and caspase-3 activation and augmented Hsp70 upregulation. Moreover, a peptide containing the Cav-1 scaffold domain sequence markedly inhibited bystander apoptosis and apoptotic signal pathways. Our studies shed new light on the potential role of Cav-1 in limiting HIV pathogenesis and the development of a novel therapeutic strategy in treating HIV-1-infected patients.
Figures






Similar articles
-
Site-specific mutations in HIV-1 gp41 reveal a correlation between HIV-1-mediated bystander apoptosis and fusion/hemifusion.J Biol Chem. 2007 Jun 8;282(23):16899-906. doi: 10.1074/jbc.M701701200. Epub 2007 Apr 6. J Biol Chem. 2007. PMID: 17416587
-
Anti-HIV-1 activity of enfuvirtide (T-20) by inhibition of bystander cell death.Antivir Ther. 2003 Apr;8(2):155-61. Antivir Ther. 2003. PMID: 12741628
-
Host and Viral Factors in HIV-Mediated Bystander Apoptosis.Viruses. 2017 Aug 22;9(8):237. doi: 10.3390/v9080237. Viruses. 2017. PMID: 28829402 Free PMC article. Review.
-
Single amino acid change in gp41 region of HIV-1 alters bystander apoptosis and CD4 decline in humanized mice.Virol J. 2011 Jan 21;8:34. doi: 10.1186/1743-422X-8-34. Virol J. 2011. PMID: 21255440 Free PMC article.
-
Role of HIV Gp41 mediated fusion/hemifusion in bystander apoptosis.Cell Mol Life Sci. 2008 Oct;65(20):3134-44. doi: 10.1007/s00018-008-8147-6. Cell Mol Life Sci. 2008. PMID: 18500445 Free PMC article. Review.
Cited by
-
Caveolin-1 plays a critical role in host immunity against Klebsiella pneumoniae by regulating STAT5 and Akt activity.Eur J Immunol. 2012 Jun;42(6):1500-11. doi: 10.1002/eji.201142051. Eur J Immunol. 2012. PMID: 22678904 Free PMC article.
-
The HR2 polymorphism N140I in the HIV-1 gp41 combined with the HR1 V38A mutation is associated with a less cytopathic phenotype.Retrovirology. 2012 Feb 14;9:15. doi: 10.1186/1742-4690-9-15. Retrovirology. 2012. PMID: 22333046 Free PMC article.
-
Caveolin-1 Promotes the Imbalance of Th17/Treg in Chronic Obstructive Pulmonary Disease by Regulating Hsp70 Expression.Int J Chron Obstruct Pulmon Dis. 2023 Apr 12;18:565-574. doi: 10.2147/COPD.S398780. eCollection 2023. Int J Chron Obstruct Pulmon Dis. 2023. PMID: 37077366 Free PMC article.
-
Caveolin-1 reduces HIV-1 infectivity by restoration of HIV Nef mediated impairment of cholesterol efflux by apoA-I.Retrovirology. 2012 Oct 15;9:85. doi: 10.1186/1742-4690-9-85. Retrovirology. 2012. PMID: 23067370 Free PMC article.
-
Identification of molecular sub-networks associated with cell survival in a chronically SIVmac-infected human CD4+ T cell line.Virol J. 2014 Aug 27;11:152. doi: 10.1186/1743-422X-11-152. Virol J. 2014. PMID: 25163480 Free PMC article.
References
-
- Ameisen, J. C. 1998. HIV. Setting death in motion. Nature 395:117-119. - PubMed
-
- Ameisen, J. C., and A. Capron. 1991. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol. Today 12:102-105. - PubMed
-
- Ameisen, J. C., J. Estaquier, T. Idziorek, and F. De Bels. 1995. Programmed cell death and AIDS pathogenesis: significance and potential mechanisms. Curr. Top. Microbiol. Immunol. 200:195-211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

