Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis
- PMID: 20383141
- PMCID: PMC3855841
- DOI: 10.1038/ncb2047
Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis
Abstract
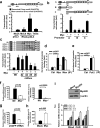
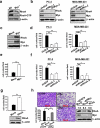
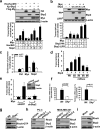
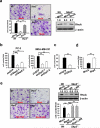
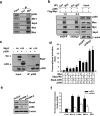
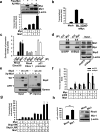
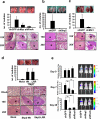
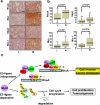
The RhoA GTPase is crucial in numerous biological functions and is linked to cancer metastasis. However, the understanding of the molecular mechanism responsible for RhoA transcription is still very limited. Here we show that RhoA transcription is orchestrated by the Myc-Skp2-Miz1-p300 transcriptional complex. Skp2 cooperates with Myc to induce RhoA transcription by recruiting Miz1 and p300 to the RhoA promoter independently of Skp1-Cullin-F-box protein containing complex (SCF)-Skp2 E3 ligase activity. Deficiency of this complex results in impairment in RhoA expression, cell migration, invasion, and breast cancer metastasis, recapitulating the phenotypes observed in RhoA knockdown, and RhoA restoration rescues the defect in cell invasion. Overexpression of the Myc-Skp2-Miz1 complex is found in metastatic human cancers and is correlated with RhoA expression. Our study provides insight into how oncogenic Skp2 and Myc coordinate to induce RhoA transcription and establishes a novel SCF-Skp2 E3-ligase-independent function for oncogenic Skp2 in transcription and cancer metastasis.
Figures
Similar articles
-
Association of human CUL-1 and ubiquitin-conjugating enzyme CDC34 with the F-box protein p45(SKP2): evidence for evolutionary conservation in the subunit composition of the CDC34-SCF pathway.EMBO J. 1998 Jan 15;17(2):368-83. doi: 10.1093/emboj/17.2.368. EMBO J. 1998. PMID: 9430629 Free PMC article.
-
Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate.Proc Natl Acad Sci U S A. 2006 Aug 1;103(31):11515-20. doi: 10.1073/pnas.0603921103. Epub 2006 Jul 21. Proc Natl Acad Sci U S A. 2006. PMID: 16861300 Free PMC article.
-
Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB.Nat Cell Biol. 2009 Apr;11(4):420-32. doi: 10.1038/ncb1849. Epub 2009 Mar 8. Nat Cell Biol. 2009. PMID: 19270694 Free PMC article.
-
Implication of the ubiquitin/proteasome system in Myc-regulated transcription.Cell Cycle. 2003 Sep-Oct;2(5):403-7. Cell Cycle. 2003. PMID: 12963825 Review.
-
Targeting SCF E3 Ligases for Cancer Therapies.Adv Exp Med Biol. 2020;1217:123-146. doi: 10.1007/978-981-15-1025-0_9. Adv Exp Med Biol. 2020. PMID: 31898226 Review.
Cited by
-
Investigation of the impact of bromodomain inhibition on cytoskeleton stability and contraction.bioRxiv [Preprint]. 2023 Nov 14:2023.11.14.567076. doi: 10.1101/2023.11.14.567076. bioRxiv. 2023. Update in: Cell Commun Signal. 2024 Mar 16;22(1):184. doi: 10.1186/s12964-024-01553-6. PMID: 38014184 Free PMC article. Updated. Preprint.
-
Down-regulation of Skp2 expression inhibits invasion and lung metastasis in osteosarcoma.Sci Rep. 2018 Sep 24;8(1):14294. doi: 10.1038/s41598-018-32428-9. Sci Rep. 2018. PMID: 30250282 Free PMC article.
-
HNRNPC regulates RhoA to induce DNA damage repair and cancer-associated fibroblast activation causing radiation resistance in pancreatic cancer.J Cell Mol Med. 2022 Apr;26(8):2322-2336. doi: 10.1111/jcmm.17254. Epub 2022 Mar 11. J Cell Mol Med. 2022. PMID: 35277915 Free PMC article.
-
The 11S Proteasome Subunit PSME3 Is a Positive Feedforward Regulator of NF-κB and Important for Host Defense against Bacterial Pathogens.Cell Rep. 2016 Feb 2;14(4):737-749. doi: 10.1016/j.celrep.2015.12.069. Epub 2016 Jan 14. Cell Rep. 2016. PMID: 26776519 Free PMC article.
-
Regulation of c-Myc protein stability by proteasome activator REGγ.Cell Death Differ. 2015 Jun;22(6):1000-11. doi: 10.1038/cdd.2014.188. Epub 2014 Nov 21. Cell Death Differ. 2015. PMID: 25412630 Free PMC article.
References
-
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. - PubMed
-
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. - PubMed
-
- Moscow JA, et al. Examination of human tumors for rhoA mutations. Oncogene. 1994;9:189–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

