Actin and phosphoinositide recruitment to fully formed Candida albicans phagosomes in mouse macrophages
- PMID: 20375582
- PMCID: PMC3005358
- DOI: 10.1159/000173694
Actin and phosphoinositide recruitment to fully formed Candida albicans phagosomes in mouse macrophages
Abstract
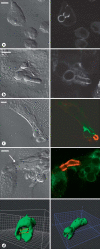
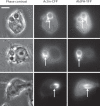
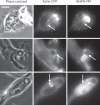
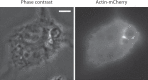
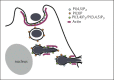
Candida albicans is a dimorphic yeast that enters macrophages (Mphi) via the beta-glucan receptor dectin-1. Phagocytosis of C. albicans is characterized by actin polymerization, Syk kinase activation and rapid acquisition of phagolysosomal markers. In mice, C. albicans are able to resist the harsh environment of the phagosome and form pseudohyphae inside the phagolysosomal compartment, eventually extending from the Mphi. In this study, we investigated these unique C. albicans phagosomes and found that actin localized dynamically around the phagosomes, before disintegrating. Membrane phosphoinositides, PI(4,5)P(2), PI(3,4,5)P(3), PI(3,4)P(2), and PI(3)P also localized to the phagosomes. Localization was not related to actin polymerization, and inhibitor studies showed that polymerization of actin on the C. albicans phagosome was independent of PI3K. The ability of mature C. albicans phagosomes to stimulate actin polymerization could facilitate the escape of the growing yeast from the Mphi.
Copyright 2008 S. Karger AG, Basel.
Figures
Similar articles
-
Candida albicans hypha formation and mannan masking of β-glucan inhibit macrophage phagosome maturation.mBio. 2014 Dec 2;5(6):e01874. doi: 10.1128/mBio.01874-14. mBio. 2014. PMID: 25467440 Free PMC article.
-
Rab14 regulates maturation of macrophage phagosomes containing the fungal pathogen Candida albicans and outcome of the host-pathogen interaction.Infect Immun. 2015 Apr;83(4):1523-35. doi: 10.1128/IAI.02917-14. Epub 2015 Feb 2. Infect Immun. 2015. PMID: 25644001 Free PMC article.
-
SHIP-1 Couples to the Dectin-1 hemITAM and Selectively Modulates Reactive Oxygen Species Production in Dendritic Cells in Response to Candida albicans.J Immunol. 2015 Nov 1;195(9):4466-4478. doi: 10.4049/jimmunol.1402874. Epub 2015 Sep 28. J Immunol. 2015. PMID: 26416276 Free PMC article.
-
Phosphoinositides signaling modulates microglial actin remodeling and phagocytosis in Alzheimer's disease.Cell Commun Signal. 2021 Feb 24;19(1):28. doi: 10.1186/s12964-021-00715-0. Cell Commun Signal. 2021. PMID: 33627135 Free PMC article. Review.
-
Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(R)ome.Curr Opin Cell Biol. 2004 Aug;16(4):422-8. doi: 10.1016/j.ceb.2004.06.006. Curr Opin Cell Biol. 2004. PMID: 15261675 Review.
Cited by
-
Novel insights into host-fungal pathogen interactions derived from live-cell imaging.Semin Immunopathol. 2015 Mar;37(2):131-9. doi: 10.1007/s00281-014-0463-3. Epub 2014 Nov 15. Semin Immunopathol. 2015. PMID: 25398200 Free PMC article. Review.
-
Proteinous Components of Neutrophil Extracellular Traps Are Arrested by the Cell Wall Proteins of Candida albicans during Fungal Infection, and Can Be Used in the Host Invasion.Cells. 2021 Oct 13;10(10):2736. doi: 10.3390/cells10102736. Cells. 2021. PMID: 34685715 Free PMC article.
-
Phagosomal F-Actin Retention by Cryptococcus gattii Induces Dendritic Cell Immunoparalysis.mBio. 2020 Nov 24;11(6):e01821-20. doi: 10.1128/mBio.01821-20. mBio. 2020. PMID: 33234684 Free PMC article.
-
Visualizing toll-like receptor-dependent phagosomal dynamics in murine dendritic cells using live cell microscopy.Methods Mol Biol. 2015;1270:191-203. doi: 10.1007/978-1-4939-2309-0_15. Methods Mol Biol. 2015. PMID: 25702119 Free PMC article.
-
Immune cells fold and damage fungal hyphae.Proc Natl Acad Sci U S A. 2021 Apr 13;118(15):e2020484118. doi: 10.1073/pnas.2020484118. Proc Natl Acad Sci U S A. 2021. PMID: 33876755 Free PMC article.
References
-
- Haynes K. Virulence in Candida species. Trends Microbiol. 2001;9:591–596. - PubMed
-
- Heinsbroek SE, Brown GD, Gordon S. Dectin-1 escape by fungal dimorphism. Trends Immunol. 2005;26:352–354. - PubMed
-
- Netea MG, van Der Graaf CA, Vonk AG, Verschueren I, van Der Meer JW, Kullberg BJ. The role of Toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis. 2002;185:1483–1489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI35950/AI/NIAID NIH HHS/United States
- R01 AI064668/AI/NIAID NIH HHS/United States
- R01 AI035950-15/AI/NIAID NIH HHS/United States
- R21 AI035950/AI/NIAID NIH HHS/United States
- 070579/WT_/Wellcome Trust/United Kingdom
- 070018/WT_/Wellcome Trust/United Kingdom
- AI64668/AI/NIAID NIH HHS/United States
- R01 AI035950/AI/NIAID NIH HHS/United States
- G0500623/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- R01 AI064668-03/AI/NIAID NIH HHS/United States
- G0601617/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

