Membrane lipids influence protein complex assembly-disassembly
- PMID: 20373736
- PMCID: PMC2862647
- DOI: 10.1021/ja101574d
Membrane lipids influence protein complex assembly-disassembly
Abstract
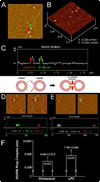
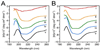
Approximately 11% smaller t-/v-SNARE ring complexes are generated using 50 nm cholesterol-associated vesicles as opposed to vesicles containing L-alpha-lysophosphatidylcholine (LPC), as observed using atomic force microscopy. Circular dichroism spectroscopy demonstrated that in the presence of LPC as opposed to cholesterol, N-ethylmaleimide-sensitive factor + adenosine triphosphate induces disassembly of beta-sheet structures but not the alpha-helical contents within the t-/v-SNARE complex.
Figures
Similar articles
-
Lysophosphatidylcholine inhibits membrane-associated SNARE complex disassembly.J Cell Mol Med. 2012 Aug;16(8):1701-8. doi: 10.1111/j.1582-4934.2011.01433.x. J Cell Mol Med. 2012. PMID: 21883893 Free PMC article.
-
Energy-dependent disassembly of self-assembled SNARE complex: observation at nanometer resolution using atomic force microscopy.J Am Chem Soc. 2006 Jan 11;128(1):26-7. doi: 10.1021/ja056286v. J Am Chem Soc. 2006. PMID: 16390104
-
Assembly and disassembly of SNAREs in membrane fusion.Methods Cell Biol. 2008;90:157-82. doi: 10.1016/S0091-679X(08)00808-X. Methods Cell Biol. 2008. PMID: 19195550
-
Role of SNAREs in membrane fusion.Adv Exp Med Biol. 2011;713:13-32. doi: 10.1007/978-94-007-0763-4_3. Adv Exp Med Biol. 2011. PMID: 21432012 Review.
-
SNARE complex assembly and disassembly.Curr Biol. 2018 Apr 23;28(8):R397-R401. doi: 10.1016/j.cub.2018.01.005. Curr Biol. 2018. PMID: 29689222 Review.
Cited by
-
Function Suggests Nano-Structure: Quantitative Structural Support for SNARE-Mediated Pore Formation.Neurotox Res. 2016 Jan;29(1):1-9. doi: 10.1007/s12640-015-9559-3. Epub 2015 Sep 25. Neurotox Res. 2016. PMID: 26407673
-
Neuronal Porosome Complex: Secretory Machinery at the Nerve Terminal.Discoveries (Craiova). 2017 Jul 28;5(3):e77. doi: 10.15190/d.2017.7. Discoveries (Craiova). 2017. PMID: 32309595 Free PMC article. Review.
-
'Porosome' discovered nearly 20 years ago provides molecular insights into the kiss-and-run mechanism of cell secretion.J Cell Mol Med. 2015 Jul;19(7):1427-40. doi: 10.1111/jcmm.12598. Epub 2015 May 28. J Cell Mol Med. 2015. PMID: 26033351 Free PMC article. Review.
-
3D organization and function of the cell: Golgi budding and vesicle biogenesis to docking at the porosome complex.Histochem Cell Biol. 2012 Jun;137(6):703-18. doi: 10.1007/s00418-012-0948-x. Epub 2012 Apr 13. Histochem Cell Biol. 2012. PMID: 22527693
-
Phospholipases A2 and neural membrane dynamics: implications for Alzheimer's disease.J Neurochem. 2011 Mar;116(5):813-9. doi: 10.1111/j.1471-4159.2010.07033.x. Epub 2011 Jan 7. J Neurochem. 2011. PMID: 21214562 Free PMC article. Review.
References
-
- Bennett K, Calakos N, Scheller RH. Science. 1992;257:255–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

