Genetic, structural, and antigenic analyses of glycan diversity in the O-linked protein glycosylation systems of human Neisseria species
- PMID: 20363948
- PMCID: PMC2876500
- DOI: 10.1128/JB.00101-10
Genetic, structural, and antigenic analyses of glycan diversity in the O-linked protein glycosylation systems of human Neisseria species
Abstract
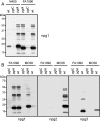
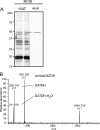
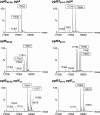
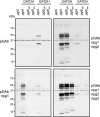
Bacterial capsular polysaccharides and lipopolysaccharides are well-established ligands of innate and adaptive immune effectors and often exhibit structural and antigenic variability. Although many surface-localized glycoproteins have been identified in bacterial pathogens and symbionts, it not clear if and how selection impacts associated glycoform structure. Here, a systematic approach was devised to correlate gene repertoire with protein-associated glycoform structure in Neisseria species important to human health and disease. By manipulating the protein glycosylation (pgl) gene content and assessing the glycan structure by mass spectrometry and reactivity with monoclonal antibodies, it was established that protein-associated glycans are antigenically variable and that at least nine distinct glycoforms can be expressed in vitro. These studies also revealed that in addition to Neisseria gonorrhoeae strain N400, one other gonococcal strain and isolates of Neisseria meningitidis and Neisseria lactamica exhibit broad-spectrum O-linked protein glycosylation. Although a strong correlation between pgl gene content, glycoform expression, and serological profile was observed, there were significant exceptions, particularly with regard to levels of microheterogeneity. This work provides a technological platform for molecular serotyping of neisserial protein glycans and for elucidating pgl gene evolution.
Figures




Similar articles
-
Genetic, Functional, and Immunogenic Analyses of the O-Linked Protein Glycosylation System in Neisseria meningitidis Serogroup A ST-7 Isolates.J Bacteriol. 2023 Mar 21;205(3):e0045822. doi: 10.1128/jb.00458-22. Epub 2023 Feb 28. J Bacteriol. 2023. PMID: 36852982 Free PMC article.
-
Genotypic and Phenotypic Characterization of the O-Linked Protein Glycosylation System Reveals High Glycan Diversity in Paired Meningococcal Carriage Isolates.J Bacteriol. 2018 Jul 25;200(16):e00794-17. doi: 10.1128/JB.00794-17. Print 2018 Aug 15. J Bacteriol. 2018. PMID: 29555702 Free PMC article.
-
Characterization of a Unique Tetrasaccharide and Distinct Glycoproteome in the O-Linked Protein Glycosylation System of Neisseria elongata subsp. glycolytica.J Bacteriol. 2015 Oct 19;198(2):256-67. doi: 10.1128/JB.00620-15. Print 2016 Jan 15. J Bacteriol. 2015. PMID: 26483525 Free PMC article.
-
The role of pilin glycan in neisserial pathogenesis.Mol Cell Biochem. 2003 Nov;253(1-2):179-90. doi: 10.1023/a:1026058311857. Mol Cell Biochem. 2003. PMID: 14619968 Review.
-
Pathogenic consequences of Neisseria gonorrhoeae pilin glycan variation.Microbes Infect. 2004 Jun;6(7):693-701. doi: 10.1016/j.micinf.2004.02.019. Microbes Infect. 2004. PMID: 15158777 Review.
Cited by
-
Genetic determinants of genus-level glycan diversity in a bacterial protein glycosylation system.PLoS Genet. 2019 Dec 23;15(12):e1008532. doi: 10.1371/journal.pgen.1008532. eCollection 2019 Dec. PLoS Genet. 2019. PMID: 31869330 Free PMC article.
-
Sticky and sweet: the role of post-translational modifications on neisserial pili.Front Microbiol. 2011 Apr 27;2:87. doi: 10.3389/fmicb.2011.00087. eCollection 2011. Front Microbiol. 2011. PMID: 21779276 Free PMC article. No abstract available.
-
Sculpting the Bacterial O-Glycoproteome: Functional Analyses of Orthologous Oligosaccharyltransferases with Diverse Targeting Specificities.mBio. 2022 Jun 28;13(3):e0379721. doi: 10.1128/mbio.03797-21. Epub 2022 Apr 26. mBio. 2022. PMID: 35471082 Free PMC article.
-
The renaissance of bacillosamine and its derivatives: pathway characterization and implications in pathogenicity.Biochemistry. 2014 Feb 4;53(4):624-38. doi: 10.1021/bi401546r. Epub 2014 Jan 21. Biochemistry. 2014. PMID: 24383882 Free PMC article. Review.
-
Hypomorphic glycosyltransferase alleles and recoding at contingency loci influence glycan microheterogeneity in the protein glycosylation system of Neisseria species.J Bacteriol. 2012 Sep;194(18):5034-43. doi: 10.1128/JB.00950-12. Epub 2012 Jul 13. J Bacteriol. 2012. PMID: 22797763 Free PMC article.
References
-
- Aas, F. E., W. Egge-Jacobsen, H. C. Winther-Larsen, C. Lovold, P. G. Hitchen, A. Dell, and M. Koomey. 2006. Neisseria gonorrhoeae type IV pili undergo multisite, hierarchical modifications with phosphoethanolamine and phosphocholine requiring an enzyme structurally related to lipopolysaccharide phosphoethanolamine transferases. J. Biol. Chem. 281:27712-27723. - PubMed
-
- Aas, F. E., H. C. Winther-Larsen, M. Wolfgang, S. Frye, C. Lovold, N. Roos, J. P. van Putten, and M. Koomey. 2007. Substitutions in the N-terminal alpha helical spine of Neisseria gonorrhoeae pilin affect type IV pilus assembly, dynamics and associated functions. Mol. Microbiol. 63:69-85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

