Multiple faces of the SAGA complex
- PMID: 20363118
- PMCID: PMC2900470
- DOI: 10.1016/j.ceb.2010.03.005
Multiple faces of the SAGA complex
Abstract
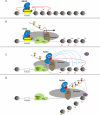
The SAGA complex provides a paradigm for multisubunit histone modifying complexes. Although first characterized as a histone acetyltransferase, because of the Gcn5 subunit, SAGA is now known to contain a second activity, a histone deubiquitinase, as well as subunits important for interactions with transcriptional activators and the general transcription machinery. The functions of SAGA in transcriptional activation are well-established in Saccharomyces cerevisiae. Recent studies in S. pombe, Drosophila, and mammalian systems reveal that SAGA also has important roles in transcript elongation, the regulation of protein stability, and telomere maintenance. These functions are essential for normal embryo development in flies and mice, and mutations or altered expression of SAGA subunits correlate with neurological disease and aggressive cancers in humans.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
The SAGA continues: The rise of cis- and trans-histone crosstalk pathways.Biochim Biophys Acta Gene Regul Mech. 2021 Feb;1864(2):194600. doi: 10.1016/j.bbagrm.2020.194600. Epub 2020 Jul 6. Biochim Biophys Acta Gene Regul Mech. 2021. PMID: 32645359 Free PMC article. Review.
-
Multifaceted activities of the plant SAGA complex.Biochim Biophys Acta Gene Regul Mech. 2021 Feb;1864(2):194613. doi: 10.1016/j.bbagrm.2020.194613. Epub 2020 Jul 31. Biochim Biophys Acta Gene Regul Mech. 2021. PMID: 32745625 Review.
-
The related coactivator complexes SAGA and ATAC control embryonic stem cell self-renewal through acetyltransferase-independent mechanisms.Cell Rep. 2021 Aug 24;36(8):109598. doi: 10.1016/j.celrep.2021.109598. Cell Rep. 2021. PMID: 34433046 Free PMC article.
-
The essential gene wda encodes a WD40 repeat subunit of Drosophila SAGA required for histone H3 acetylation.Mol Cell Biol. 2006 Oct;26(19):7178-89. doi: 10.1128/MCB.00130-06. Mol Cell Biol. 2006. PMID: 16980620 Free PMC article.
-
The SAGA chromatin-modifying complex: the sum of its parts is greater than the whole.Genes Dev. 2020 Oct 1;34(19-20):1287-1303. doi: 10.1101/gad.341156.120. Genes Dev. 2020. PMID: 33004486 Free PMC article. Review.
Cited by
-
Uncovering the role of Sgf73 in maintaining SAGA deubiquitinating module structure and activity.J Mol Biol. 2015 Apr 24;427(8):1765-78. doi: 10.1016/j.jmb.2014.12.004. Epub 2014 Dec 17. J Mol Biol. 2015. PMID: 25526805 Free PMC article.
-
Histone chaperones Nap1 and Vps75 regulate histone acetylation during transcription elongation.Mol Cell Biol. 2013 Apr;33(8):1645-56. doi: 10.1128/MCB.01121-12. Epub 2013 Feb 11. Mol Cell Biol. 2013. PMID: 23401858 Free PMC article.
-
The Transition of Poised RNA Polymerase II to an Actively Elongating State Is a "Complex" Affair.Genet Res Int. 2011;2011:206290. doi: 10.4061/2011/206290. Epub 2011 Oct 12. Genet Res Int. 2011. PMID: 22567346 Free PMC article.
-
SAGA function in tissue-specific gene expression.Trends Cell Biol. 2012 Apr;22(4):177-84. doi: 10.1016/j.tcb.2011.11.005. Epub 2011 Dec 21. Trends Cell Biol. 2012. PMID: 22196215 Free PMC article.
-
Conformational flexibility and subunit arrangement of the modular yeast Spt-Ada-Gcn5 acetyltransferase complex.J Biol Chem. 2015 Apr 17;290(16):10057-70. doi: 10.1074/jbc.M114.624684. Epub 2015 Feb 20. J Biol Chem. 2015. PMID: 25713136 Free PMC article.
References
-
- Brownell JE, Zhou J, Ranalli T, Kobayashi R, Edmondson DG, Roth SY, Allis CD. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996;84:843–851. - PubMed
-
- Weake VM, Workman JL. Histone ubiquitination: triggering gene activity. Molecular cell. 2008;29:653–663. - PubMed
-
- Nagy Z, Tora L. Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene. 2007;26:5341–5357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

