Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species
- PMID: 20351271
- PMCID: PMC2867754
- DOI: 10.1073/pnas.1002459107
Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species
Abstract
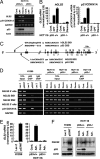
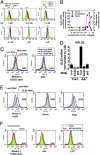
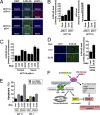
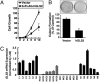
We identified a p53 target gene, phosphate-activated mitochondrial glutaminase (GLS2), a key enzyme in conversion of glutamine to glutamate, and thereby a regulator of glutathione (GSH) synthesis and energy production. GLS2 expression is induced in response to DNA damage or oxidative stress in a p53-dependent manner, and p53 associates with the GLS2 promoter. Elevated GLS2 facilitates glutamine metabolism and lowers intracellular reactive oxygen species (ROS) levels, resulting in an overall decrease in DNA oxidation as determined by measurement of 8-OH-dG content in both normal and stressed cells. Further, siRNA down-regulation of either GLS2 or p53 compromises the GSH-dependent antioxidant system and increases intracellular ROS levels. High ROS levels following GLS2 knockdown also coincide with stimulation of p53-induced cell death. We propose that GLS2 control of intracellular ROS levels and the apoptotic response facilitates the ability of p53 to protect cells from accumulation of genomic damage and allows cells to survive after mild and repairable genotoxic stress. Indeed, overexpression of GLS2 reduces the growth of tumor cells and colony formation. Further, compared with normal tissue, GLS2 expression is reduced in liver tumors. Thus, our results provide evidence for a unique metabolic role for p53, linking glutamine metabolism, energy, and ROS homeostasis, which may contribute to p53 tumor suppressor function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Alternative fuel--another role for p53 in the regulation of metabolism.Proc Natl Acad Sci U S A. 2010 Apr 20;107(16):7117-8. doi: 10.1073/pnas.1002656107. Epub 2010 Apr 14. Proc Natl Acad Sci U S A. 2010. PMID: 20393124 Free PMC article. No abstract available.
-
Journal club. A cancer biologist weighs up p53, metabolism and cancer.Nature. 2010 Aug 19;466(7309):905. doi: 10.1038/466905d. Nature. 2010. PMID: 20725003 No abstract available.
Similar articles
-
Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function.Proc Natl Acad Sci U S A. 2010 Apr 20;107(16):7455-60. doi: 10.1073/pnas.1001006107. Epub 2010 Apr 8. Proc Natl Acad Sci U S A. 2010. PMID: 20378837 Free PMC article.
-
Knock-down of glutaminase 2 expression decreases glutathione, NADH, and sensitizes cervical cancer to ionizing radiation.Biochim Biophys Acta. 2013 Dec;1833(12):2996-3005. doi: 10.1016/j.bbamcr.2013.08.003. Epub 2013 Aug 13. Biochim Biophys Acta. 2013. PMID: 23954443
-
Both GLS silencing and GLS2 overexpression synergize with oxidative stress against proliferation of glioma cells.J Mol Med (Berl). 2014 Mar;92(3):277-90. doi: 10.1007/s00109-013-1105-2. Epub 2013 Nov 26. J Mol Med (Berl). 2014. PMID: 24276018 Free PMC article.
-
Glutaminases regulate glutathione and oxidative stress in cancer.Arch Toxicol. 2020 Aug;94(8):2603-2623. doi: 10.1007/s00204-020-02838-8. Epub 2020 Jul 18. Arch Toxicol. 2020. PMID: 32681190 Review.
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article. Review.
Cited by
-
The tangled circuitry of metabolism and apoptosis.Mol Cell. 2013 Feb 7;49(3):399-410. doi: 10.1016/j.molcel.2012.12.026. Mol Cell. 2013. PMID: 23395270 Free PMC article. Review.
-
The complexity of p53-mediated metabolic regulation in tumor suppression.Semin Cancer Biol. 2022 Oct;85:4-32. doi: 10.1016/j.semcancer.2021.03.010. Epub 2021 Mar 27. Semin Cancer Biol. 2022. PMID: 33785447 Free PMC article. Review.
-
Metabolic regulation of oxygen and redox homeostasis by p53: lessons from evolutionary biology?Free Radic Biol Med. 2012 Sep 15;53(6):1279-85. doi: 10.1016/j.freeradbiomed.2012.07.026. Epub 2012 Jul 25. Free Radic Biol Med. 2012. PMID: 22841759 Free PMC article. Review.
-
Iron accumulation and lipid peroxidation: implication of ferroptosis in hepatocellular carcinoma.Front Endocrinol (Lausanne). 2024 Jan 4;14:1319969. doi: 10.3389/fendo.2023.1319969. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38274225 Free PMC article. Review.
-
PTEN in cancer, metabolism, and aging.Trends Endocrinol Metab. 2013 Apr;24(4):184-9. doi: 10.1016/j.tem.2012.11.002. Epub 2012 Dec 12. Trends Endocrinol Metab. 2013. PMID: 23245767 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

