Caspases in virus-infected cells contribute to recognition by CD8+ T lymphocytes
- PMID: 20348426
- PMCID: PMC4202692
- DOI: 10.4049/jimmunol.1000050
Caspases in virus-infected cells contribute to recognition by CD8+ T lymphocytes
Abstract
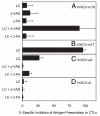
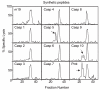
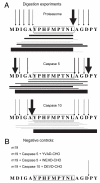
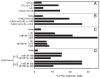
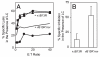
CD8(+) cytotoxic T lymphocytes recognize infected cells in which MHC class I molecules present pathogen-derived peptides that have been processed mainly by proteasomes. Many infections induce a set of proteases, the caspases involved in apoptosis or inflammation. In this study, we report that processing and presentation of a short vaccinia virus-encoded Ag can take place also by a nonproteasomal pathway, which was blocked in infected cells with chemical inhibitors of caspases. By cleaving at noncanonical sites, at least two caspases generated antigenic peptides recognized by T lymphocytes. The sites and the peptidic products were partially overlapping but different to those used and produced by proteasomes in vitro. Antigenic natural peptides produced in infected cells by either pathway were quantitatively and qualitatively similar. Finally, coexpression of the natural vaccinia virus protein B13, which is an inhibitor of caspases and apoptosis, impaired Ag presentation by the caspase pathway in infected cells. These data support the hypothesis that numerous cellular proteolytic systems, including those induced during infection, such as caspases involved in apoptosis or in inflammation, contribute to the repertoire of presented peptides, thereby facilitating immunosurveillance.
Figures

Similar articles
-
Endoplasmic reticulum aminopeptidase associated with antigen processing defines the composition and structure of MHC class I peptide repertoire in normal and virus-infected cells.J Immunol. 2010 Mar 15;184(6):3033-42. doi: 10.4049/jimmunol.0903712. Epub 2010 Feb 19. J Immunol. 2010. PMID: 20173027 Free PMC article.
-
Need for tripeptidyl-peptidase II in major histocompatibility complex class I viral antigen processing when proteasomes are detrimental.J Biol Chem. 2006 Dec 29;281(52):39925-34. doi: 10.1074/jbc.M608522200. Epub 2006 Nov 6. J Biol Chem. 2006. PMID: 17088258
-
Furin-processed antigens targeted to the secretory route elicit functional TAP1-/-CD8+ T lymphocytes in vivo.J Immunol. 2009 Oct 1;183(7):4639-47. doi: 10.4049/jimmunol.0901356. Epub 2009 Sep 14. J Immunol. 2009. PMID: 19752221
-
Multiple proteases process viral antigens for presentation by MHC class I molecules to CD8(+) T lymphocytes.Mol Immunol. 2002 Oct;39(3-4):235-47. doi: 10.1016/s0161-5890(02)00104-9. Mol Immunol. 2002. PMID: 12200053 Review.
-
Generation of MHC class I ligands in the secretory and vesicular pathways.Cell Mol Life Sci. 2011 May;68(9):1543-52. doi: 10.1007/s00018-011-0661-2. Epub 2011 Mar 9. Cell Mol Life Sci. 2011. PMID: 21387141 Free PMC article. Review.
Cited by
-
Engineering of double recombinant vaccinia virus with enhanced oncolytic potential for solid tumor virotherapy.Oncotarget. 2016 Nov 8;7(45):74171-74188. doi: 10.18632/oncotarget.12367. Oncotarget. 2016. PMID: 27708236 Free PMC article.
-
Allele-dependent processing pathways generate the endogenous human leukocyte antigen (HLA) class I peptide repertoire in transporters associated with antigen processing (TAP)-deficient cells.J Biol Chem. 2011 Nov 4;286(44):38054-38059. doi: 10.1074/jbc.M111.281808. Epub 2011 Sep 13. J Biol Chem. 2011. PMID: 21914809 Free PMC article.
-
Concerted in vitro trimming of viral HLA-B27-restricted ligands by human ERAP1 and ERAP2 aminopeptidases.PLoS One. 2013 Nov 1;8(11):e79596. doi: 10.1371/journal.pone.0079596. eCollection 2013. PLoS One. 2013. PMID: 24223975 Free PMC article.
-
Polyfunctional type-1, -2, and -17 CD8⁺ T cell responses to apoptotic self-antigens correlate with the chronic evolution of hepatitis C virus infection.PLoS Pathog. 2012;8(6):e1002759. doi: 10.1371/journal.ppat.1002759. Epub 2012 Jun 21. PLoS Pathog. 2012. PMID: 22737070 Free PMC article.
-
Increased CD8+ T cell responses to apoptotic T cell-associated antigens in multiple sclerosis.J Neuroinflammation. 2013 Jul 27;10:94. doi: 10.1186/1742-2094-10-94. J Neuroinflammation. 2013. PMID: 23890271 Free PMC article.
References
-
- Kloetzel PM, Ossendorp F. Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr. Opin. Immunol. 2004;16:76–81. - PubMed
-
- Rock KL, York IA, Goldberg AL. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat. Immunol. 2004;5:670–677. - PubMed
-
- Hammer GE, Kanaseki T, Shastri N. The final touches make perfect the peptide-MHC class I repertoire. Immunity. 2007;26:397–406. - PubMed
-
- Del Val M, López D. Multiple proteases process viral antigens for presentation by MHC class I molecules to CD8 T lymphocytes. Mol. Immunol. 2002;39:235–247. - PubMed
-
- Seifert U, Marañón C, Shmueli A, Desoutter JF, Wesoloski L, Janek K, Henklein P, Diescher S, Andrieu M, de la Salle H, et al. An essential role for tripeptidyl peptidase in the generation of an MHC class I epitope. Nat. Immunol. 2003;4:375–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

