Multivalent dendrimeric and monomeric adenosine agonists attenuate cell death in HL-1 mouse cardiomyocytes expressing the A(3) receptor
- PMID: 20346920
- PMCID: PMC2880883
- DOI: 10.1016/j.bcp.2010.03.020
Multivalent dendrimeric and monomeric adenosine agonists attenuate cell death in HL-1 mouse cardiomyocytes expressing the A(3) receptor
Abstract
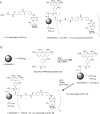
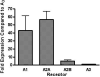
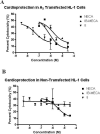
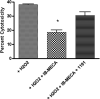
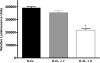
Multivalent dendrimeric conjugates of GPCR ligands may have increased potency or selectivity in comparison to monomeric ligands, a phenomenon that was tested in a model of cytoprotection in mouse HL-1 cardiomyocytes. Quantitative RT-PCR indicated high expression levels of endogenous A(1) and A(2A) adenosine receptors (ARs), but not of A(2B) and A(3)ARs. Activation of the heterologously expressed human A(3)AR in HL-1 cells by AR agonists significantly attenuated cell damage following 4h exposure to H(2)O(2) (750 microM) but not in untransfected cells. The A(3) agonist IB-MECA (EC(50) 3.8 microM) and the non-selective agonist NECA (EC(50) 3.9 microM) protected A(3) AR-transfected cells against H(2)O(2) in a concentration-dependent manner, as determined by lactate dehydrogenase release. A generation 5.5 PAMAM (polyamidoamine) dendrimeric conjugate of a N(6)-chain-functionalized adenosine agonist was synthesized and its mass indicated an average of 60 amide-linked nucleoside moieties out of 256 theoretical attachment sites. It non-selectively activated the A(3)AR to inhibit forskolin-stimulated cAMP formation (IC(50) 66nM) and, similarly, protected A(3)-transfected HL-1 cells from apoptosis-inducing H(2)O(2) with greater potency (IC(50) 35nM) than monomeric nucleosides. Thus, a PAMAM conjugate retained AR binding affinity and displayed greatly enhanced cardioprotective potency.
Published by Elsevier Inc.
Figures
Similar articles
-
Anti-ischemic effects of multivalent dendrimeric A₃ adenosine receptor agonists in cultured cardiomyocytes and in the isolated rat heart.Pharmacol Res. 2012 Mar;65(3):338-46. doi: 10.1016/j.phrs.2011.11.013. Epub 2011 Dec 1. Pharmacol Res. 2012. PMID: 22154845 Free PMC article.
-
Human adenosine A(1), A(2A), A(2B), and A(3) receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2.Mol Pharmacol. 2000 Sep;58(3):477-82. Mol Pharmacol. 2000. PMID: 10953039
-
Synthesis and adenosine receptor affinity and potency of 8-alkynyl derivatives of adenosine.Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):1153-7. doi: 10.1081/NCN-100002509. Nucleosides Nucleotides Nucleic Acids. 2001. PMID: 11562976
-
Adenosine A(2A) and A(2B) receptors in cultured human and porcine coronary artery endothelial cells.Am J Physiol Heart Circ Physiol. 2000 Aug;279(2):H650-6. doi: 10.1152/ajpheart.2000.279.2.H650. Am J Physiol Heart Circ Physiol. 2000. PMID: 10924064
-
Comparative pharmacology of human adenosine receptor subtypes - characterization of stably transfected receptors in CHO cells.Naunyn Schmiedebergs Arch Pharmacol. 1998 Jan;357(1):1-9. doi: 10.1007/pl00005131. Naunyn Schmiedebergs Arch Pharmacol. 1998. PMID: 9459566
Cited by
-
Conditioned medium from hypoxic cells protects cardiomyocytes against ischemia.Mol Cell Biochem. 2012 Apr;363(1-2):167-78. doi: 10.1007/s11010-011-1169-7. Epub 2011 Dec 8. Mol Cell Biochem. 2012. PMID: 22160856
-
GPCR ligand dendrimer (GLiDe) conjugates: adenosine receptor interactions of a series of multivalent xanthine antagonists.Bioconjug Chem. 2011 Jun 15;22(6):1115-27. doi: 10.1021/bc1005812. Epub 2011 May 12. Bioconjug Chem. 2011. PMID: 21539392 Free PMC article.
-
Anti-ischemic effects of multivalent dendrimeric A₃ adenosine receptor agonists in cultured cardiomyocytes and in the isolated rat heart.Pharmacol Res. 2012 Mar;65(3):338-46. doi: 10.1016/j.phrs.2011.11.013. Epub 2011 Dec 1. Pharmacol Res. 2012. PMID: 22154845 Free PMC article.
-
GPCR ligand-dendrimer (GLiDe) conjugates: future smart drugs?Trends Pharmacol Sci. 2010 Dec;31(12):575-9. doi: 10.1016/j.tips.2010.09.002. Epub 2010 Oct 18. Trends Pharmacol Sci. 2010. PMID: 20961625 Free PMC article.
-
Programmable nanoscaffolds that control ligand display to a G-protein-coupled receptor in membranes to allow dissection of multivalent effects.J Am Chem Soc. 2014 Sep 3;136(35):12296-303. doi: 10.1021/ja504288s. Epub 2014 Aug 25. J Am Chem Soc. 2014. PMID: 25116377 Free PMC article.
References
-
- Madi L, Cohen S, Ochayin A, Bar-Yehuda S, Barer F, Fishman P. Overexpression of A3 adenosine receptor in peripheral blood mononuclear cells in rheumatoid arthritis: involvement of nuclear factor-kappaB in mediating receptor level. J Rheumatol. 2007;34:20–6. - PubMed
-
- Bar-Yehuda S, Silverman MH, Kerns WD, Ochaion A, Cohen S, Fishman P. The anti-inflammatory effect of A3 adenosine receptor agonists: a novel targeted therapy for rheumatoid arthritis. Expert Opin Investig Drugs. 2007;16:1601–13. - PubMed
-
- Silverman MH, Strand V, Markovits D, Nahir M, Reitblat T, Molad Y, et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. J Rheumatol. 2008;35:41–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

