Identification of ciliary neurotrophic factor receptor alpha as a mediator of neurotoxicity induced by alpha-synuclein
- PMID: 20340160
- PMCID: PMC3013276
- DOI: 10.1002/pmic.200900745
Identification of ciliary neurotrophic factor receptor alpha as a mediator of neurotoxicity induced by alpha-synuclein
Abstract
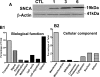
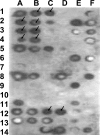
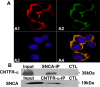
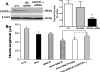
Accumulating evidence suggests that extracellular alpha-synuclein (eSNCA) plays an important role in the pathogenesis of Parkinson's disease or related synucleinopathies by inducing neurotoxicity directly or indirectly via microglial or astroglial activation. However, the mechanisms by which this occurs remain to be characterized. To explore these mechanisms, we combined three biochemical techniques - stable isotope labeling of amino acid in cell cultures (SILAC), biotin labeling of plasma membrane proteins followed by affinity purification, and analysis of unique proteins binding to SNCA peptides on membrane arrays. The SILAC proteomic analysis identified 457 proteins, of which, 245 or 172 proteins belonged to membrane or membrane associated proteins, depending on the various bioinformatics tools used for interpretation. In dopamine neuronal cells treated with eSNCA, the levels of 86 membrane proteins were increased and 35 were decreased compared with untreated cells. In peptide array analysis, 127 proteins were identified as possibly interacting with eSNCA. Of those, seven proteins were overlapped with the membrane proteins that displayed alterations in relative abundance after eSNCA treatment. One was ciliary neurotrophic factor receptor, which appeared to modulate eSNCA-mediated neurotoxicity via mechanisms related to JAK1/STAT3 signaling but independent of eSNCA endocytosis.
Figures


Similar articles
-
Rab11a and HSP90 regulate recycling of extracellular alpha-synuclein.J Neurosci. 2009 Feb 4;29(5):1480-5. doi: 10.1523/JNEUROSCI.6202-08.2009. J Neurosci. 2009. PMID: 19193894 Free PMC article.
-
Extracellular signal-regulated kinase is involved in alpha-synuclein-induced mitochondrial dynamic disorders by regulating dynamin-like protein 1.Neurobiol Aging. 2012 Dec;33(12):2841-54. doi: 10.1016/j.neurobiolaging.2012.02.001. Epub 2012 Mar 22. Neurobiol Aging. 2012. PMID: 22445325
-
Expression of ciliary neurotrophic factor (CNTF) and its tripartite receptor complex by cells of the human optic nerve head.Mol Vis. 2007 May 23;13:758-63. Mol Vis. 2007. PMID: 17563726 Free PMC article.
-
Inhibition of Microglia-Derived Oxidative Stress by Ciliary Neurotrophic Factor Protects Dopamine Neurons In Vivo from MPP⁺ Neurotoxicity.Int J Mol Sci. 2018 Nov 10;19(11):3543. doi: 10.3390/ijms19113543. Int J Mol Sci. 2018. PMID: 30423807 Free PMC article.
-
TRPV1 on astrocytes rescues nigral dopamine neurons in Parkinson's disease via CNTF.Brain. 2015 Dec;138(Pt 12):3610-22. doi: 10.1093/brain/awv297. Epub 2015 Oct 21. Brain. 2015. PMID: 26490328 Free PMC article.
Cited by
-
Cathepsin L-containing exosomes from α-synuclein-activated microglia induce neurotoxicity through the P2X7 receptor.NPJ Parkinsons Dis. 2022 Oct 6;8(1):127. doi: 10.1038/s41531-022-00394-9. NPJ Parkinsons Dis. 2022. PMID: 36202834 Free PMC article.
-
Approaches for targeted proteomics and its potential applications in neuroscience.J Biosci. 2015 Sep;40(3):607-27. doi: 10.1007/s12038-015-9537-1. J Biosci. 2015. PMID: 26333406 Review.
-
Viewing Extrinsic Proteotoxic Stress Through the Lens of Amyloid Cardiomyopathy.Physiology (Bethesda). 2016 Jul;31(4):294-9. doi: 10.1152/physiol.00047.2015. Physiology (Bethesda). 2016. PMID: 27252164 Free PMC article. Review.
-
Proteomic change by Korean Red Ginseng in the substantia nigra of a Parkinson's disease mouse model.J Ginseng Res. 2018 Oct;42(4):429-435. doi: 10.1016/j.jgr.2017.04.008. Epub 2017 Apr 28. J Ginseng Res. 2018. PMID: 30337802 Free PMC article.
References
-
- Lee SJ. Origins and effects of extracellular alpha-synuclein: implications in Parkinson's disease. J Mol Neurosci. 2008;34:17–22. - PubMed
-
- El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, et al. Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. Faseb J. 2003;17:1945–1947. - PubMed
-
- Borghi R, Marchese R, Negro A, Marinelli L, et al. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci Lett. 2000;287:65–67. - PubMed
-
- Kim YS, Lee D, Lee EK, Sung JY, et al. Multiple ligand interaction of alpha-synuclein produced various forms of protein aggregates in the presence of Abeta25–35, copper, and eosin. Brain research. 2001;908:93–98. - PubMed
-
- Chen L, Jin J, Davis J, Zhou Y, et al. Oligomeric alpha-synuclein inhibits tubulin polymerization. Biochemical and biophysical research communications. 2007;356:548–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG025327/AG/NIA NIH HHS/United States
- R01 NS057567-01A2/NS/NINDS NIH HHS/United States
- NS057567/NS/NINDS NIH HHS/United States
- R01 AG025327-01/AG/NIA NIH HHS/United States
- R01 AG033398/AG/NIA NIH HHS/United States
- R01 ES012703/ES/NIEHS NIH HHS/United States
- R01 AG033398-01/AG/NIA NIH HHS/United States
- ES016873/ES/NIEHS NIH HHS/United States
- ES012703/ES/NIEHS NIH HHS/United States
- AG033398/AG/NIA NIH HHS/United States
- R01 NS057567/NS/NINDS NIH HHS/United States
- R01 ES012703-01A1/ES/NIEHS NIH HHS/United States
- AG025327/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

