Bocavirus infection induces mitochondrion-mediated apoptosis and cell cycle arrest at G2/M phase
- PMID: 20335259
- PMCID: PMC2876597
- DOI: 10.1128/JVI.02094-09
Bocavirus infection induces mitochondrion-mediated apoptosis and cell cycle arrest at G2/M phase
Abstract
Bocavirus is a newly classified genus of the family Parvovirinae. Infection with Bocavirus minute virus of canines (MVC) produces a strong cytopathic effect in permissive Walter Reed/3873D (WRD) canine cells. We have systematically characterized the MVC infection-produced cytopathic effect in WRD cells, namely, the cell death and cell cycle arrest, and carefully examined how MVC infection induces the cytopathic effect. We found that MVC infection induces an apoptotic cell death characterized by Bax translocalization to the mitochondrial outer membrane, disruption of the mitochondrial outer membrane potential, and caspase activation. Moreover, we observed that the activation of caspases occurred only when the MVC genome was replicating, suggesting that replication of the MVC genome induces apoptosis. MVC infection also induced a gradual cell cycle arrest from the S phase in early infection to the G(2)/M phase at a later stage, which was confirmed by the upregulation of cyclin B1 and phosphorylation of cdc2. Cell cycle arrest at the G(2)/M phase was reproduced by transfection of a nonreplicative NS1 knockout mutant of the MVC infectious clone, as well as by inoculation of UV-irradiated MVC. In contrast with other parvoviruses, only expression of the MVC proteins by transfection did not induce apoptosis or cell cycle arrest. Taken together, our results demonstrate that MVC infection induces a mitochondrion-mediated apoptosis that is dependent on the replication of the viral genome, and the MVC genome per se is able to arrest the cell cycle at the G(2)/M phase. Our results may shed light on the molecular pathogenesis of Bocavirus infection in general.
Figures

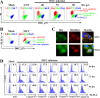
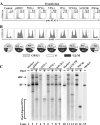
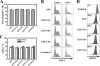
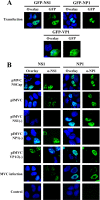
Similar articles
-
Bocavirus infection induces a DNA damage response that facilitates viral DNA replication and mediates cell death.J Virol. 2011 Jan;85(1):133-45. doi: 10.1128/JVI.01534-10. Epub 2010 Nov 3. J Virol. 2011. PMID: 21047968 Free PMC article.
-
The DNA replication, virogenesis and infection of canine minute virus in non-permissive and permissive cells.Virus Res. 2014 Jan 22;179:147-52. doi: 10.1016/j.virusres.2013.10.019. Epub 2013 Nov 12. Virus Res. 2014. PMID: 24239972
-
Oxymatrine Inhibits Bocavirus MVC Replication, Reduces Viral Gene Expression and Decreases Apoptosis Induced by Viral Infection.Virol Sin. 2019 Feb;34(1):78-87. doi: 10.1007/s12250-019-00088-2. Epub 2019 Feb 25. Virol Sin. 2019. PMID: 30805776 Free PMC article.
-
Apoptosis mechanisms induced by parvovirus infections.Acta Virol. 2022;66(2):101-109. doi: 10.4149/av_2022_210. Acta Virol. 2022. PMID: 35766467 Review.
-
[Genomic characteristics of bocavirus and molecular mechanism of its pathogenicity--a review].Wei Sheng Wu Xue Bao. 2013 May 4;53(5):421-8. Wei Sheng Wu Xue Bao. 2013. PMID: 23957145 Review. Chinese.
Cited by
-
Nuclear entry and egress of parvoviruses.Mol Microbiol. 2022 Oct;118(4):295-308. doi: 10.1111/mmi.14974. Epub 2022 Aug 24. Mol Microbiol. 2022. PMID: 35974704 Free PMC article. Review.
-
Breaking Bad: How Viruses Subvert the Cell Cycle.Front Cell Infect Microbiol. 2018 Nov 19;8:396. doi: 10.3389/fcimb.2018.00396. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30510918 Free PMC article. Review.
-
Duck hepatitis A virus type 1 mediates cell cycle arrest in the S phase.Virol J. 2022 Jun 27;19(1):111. doi: 10.1186/s12985-022-01839-6. Virol J. 2022. PMID: 35761382 Free PMC article.
-
Productive parvovirus B19 infection of primary human erythroid progenitor cells at hypoxia is regulated by STAT5A and MEK signaling but not HIFα.PLoS Pathog. 2011 Jun;7(6):e1002088. doi: 10.1371/journal.ppat.1002088. Epub 2011 Jun 16. PLoS Pathog. 2011. PMID: 21698228 Free PMC article.
-
Molecular characterization of the small nonstructural proteins of parvovirus Aleutian mink disease virus (AMDV) during infection.Virology. 2014 Mar;452-453:23-31. doi: 10.1016/j.virol.2014.01.005. Epub 2014 Jan 28. Virology. 2014. PMID: 24606679 Free PMC article.
References
-
- Abdel-Latif, L., B. K. Murray, R. L. Renberg, K. L. O'Neill, H. Porter, J. B. Jensen, and F. B. Johnson. 2006. Cell death in bovine parvovirus-infected embryonic bovine tracheal cells is mediated by necrosis rather than apoptosis. J. Gen. Virol. 87:2539-2548. - PubMed
-
- Allander, T. 2008. Human bocavirus. J. Clin. Virol. 41:29-33. - PubMed
-
- Andersen, J. L., E. S. Zimmerman, J. L. DeHart, S. Murala, O. Ardon, J. Blackett, J. Chen, and V. Planelles. 2005. ATR and GADD45alpha mediate HIV-1 Vpr-induced apoptosis. Cell Death Differ. 12:326-334. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

