Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator
- PMID: 20302940
- PMCID: PMC2862862
- DOI: 10.1016/j.nbd.2010.03.008
Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator
Abstract
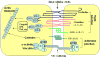
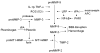
Blood-brain barrier (BBB) disruption, mediated through matrix metalloproteinases (MMPs) and other mechanisms, is a critical event during ischemic stroke. Tissue plasminogen activator (tPA) is the only FDA-approved thrombolytic therapy for acute ischemic stroke, but the efficacy and safety of its therapeutic application are limited by narrow treatment time windows and side effects. Thus, there is a pressing need to develop combinational therapy that could offset tPA side effects and improve efficacy in clinical practice. Recent experimental studies indicate that tPA has previously unidentified functions in the brain beyond its well-established thrombolytic activity, which might contribute to tPA-related side effects through MMPs (mainly MMP-9) and several signaling pathways involved in LDL receptor-related protein (LRP), activated protein C (APC) and protease-activated receptor 1 (PAR-1), platelet-derived growth factor C (PDGF-C), and N-methyl-d-aspartate (NMDA) receptor. Therapeutic targeting of MMPs and/or tPA-related signaling pathways might offer promising new approaches to combination therapies for ischemic stroke. This review provides an overview of the relationship between structural components and function of the BBB/neurovascular unit with respect to ischemic stroke. We discuss how MMPs and tPA contribute to BBB disruption during ischemic stroke and highlight recent findings of molecular signaling pathways involved in neurotoxicity of tPA therapy.
Figures

Similar articles
-
Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies.CNS Neurol Disord Drug Targets. 2008 Jun;7(3):243-53. doi: 10.2174/187152708784936608. CNS Neurol Disord Drug Targets. 2008. PMID: 18673209 Free PMC article. Review.
-
Microglial-mediated PDGF-CC activation increases cerebrovascular permeability during ischemic stroke.Acta Neuropathol. 2017 Oct;134(4):585-604. doi: 10.1007/s00401-017-1749-z. Epub 2017 Jul 19. Acta Neuropathol. 2017. PMID: 28725968 Free PMC article.
-
Therapeutic approaches to vascular protection in ischemic stroke.Acta Med Okayama. 2011 Aug;65(4):219-23. doi: 10.18926/AMO/46846. Acta Med Okayama. 2011. PMID: 21860527 Review.
-
Recombinant tissue plasminogen activator induces blood-brain barrier breakdown by a matrix metalloproteinase-9-independent pathway after transient focal cerebral ischemia in mouse.Eur J Neurosci. 2011 Oct;34(7):1085-92. doi: 10.1111/j.1460-9568.2011.07843.x. Epub 2011 Sep 6. Eur J Neurosci. 2011. PMID: 21895804
-
Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia.Stroke. 2005 Sep;36(9):1954-9. doi: 10.1161/01.STR.0000177517.01203.eb. Epub 2005 Jul 28. Stroke. 2005. PMID: 16051896
Cited by
-
ADAMTS13 exerts a thrombolytic effect in microcirculation.Thromb Haemost. 2012 Sep;108(3):527-32. doi: 10.1160/TH12-01-0046. Epub 2012 Jul 10. Thromb Haemost. 2012. PMID: 22782575 Free PMC article.
-
Towards the identification of blood biomarkers for acute stroke in humans: a comprehensive systematic review.Br J Clin Pharmacol. 2012 Aug;74(2):230-40. doi: 10.1111/j.1365-2125.2012.04212.x. Br J Clin Pharmacol. 2012. PMID: 22320313 Free PMC article. Review.
-
Neuroprotective Effect of Physical Activity in Ischemic Stroke: Focus on the Neurovascular Unit.Front Cell Neurosci. 2022 Mar 4;16:860573. doi: 10.3389/fncel.2022.860573. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35317197 Free PMC article. Review.
-
Neuroinflammatory Triangle Presenting Novel Pharmacological Targets for Ischemic Brain Injury.Front Immunol. 2021 Oct 7;12:748663. doi: 10.3389/fimmu.2021.748663. eCollection 2021. Front Immunol. 2021. PMID: 34691061 Free PMC article. Review.
-
Caspase-3 contributes to ZO-1 and Cl-5 tight-junction disruption in rapid anoxic neurovascular unit damage.PLoS One. 2011 Feb 22;6(2):e16760. doi: 10.1371/journal.pone.0016760. PLoS One. 2011. PMID: 21364989 Free PMC article.
References
-
- Abbott NJ, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- András IE, et al. The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J Cereb Blood Flow Metab. 2007;27:1431–43. - PubMed
-
- Anthony DC, et al. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–415. - PubMed
-
- Asahi M, et al. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

