Aurora kinase A induces miR-17-92 cluster through regulation of E2F1 transcription factor
- PMID: 20300951
- PMCID: PMC11115945
- DOI: 10.1007/s00018-010-0340-8
Aurora kinase A induces miR-17-92 cluster through regulation of E2F1 transcription factor
Abstract
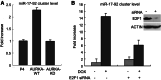
Aurora kinase A (AURKA) is an essential mitotic serine/threonine kinase and its abnormal expression is observed in many malignancies, yet the exact role for AURKA in tumorigenesis still remains elusive. Here, through a transcription factor array, we show that the transcription activity of E2F1 was increased by AURKA overexpression. Meanwhile, the E2F1 protein level was found to be upregulated and a correlation between AURKA and E2F1 expression was observed in cancer specimens. Further analysis revealed that AURKA increased E2F1 protein stability by inhibiting proteasome-dependent degradation of this protein. Additionally, a microRNA cluster, miR-17-92, was found to be upregulated upon AURKA overexpression, and this stimulation was largely repressed by E2F1 knockdown. Chromatin immunoprecipitation further demonstrated that AURKA enhanced E2F1 occupancy to the promoter of the miR-17-92 cluster. These data reveal a novel link between AURKA and microRNAs via the regulation of E2F1, providing new clues for understanding the role of AURKA in tumorigenesis.
Figures

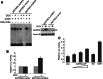



Similar articles
-
Low concentration of arsenic-induced aberrant mitosis in keratinocytes through E2F1 transcriptionally regulated Aurora-A.Toxicol Sci. 2013 Mar;132(1):43-52. doi: 10.1093/toxsci/kfs322. Epub 2012 Nov 22. Toxicol Sci. 2013. PMID: 23174854
-
The aurora kinase A regulates GSK-3beta in gastric cancer cells.Oncogene. 2009 Feb 12;28(6):866-75. doi: 10.1038/onc.2008.434. Epub 2008 Dec 8. Oncogene. 2009. PMID: 19060929 Free PMC article.
-
Reciprocal regulation of microRNA-122 and c-Myc in hepatocellular cancer: role of E2F1 and transcription factor dimerization partner 2.Hepatology. 2014 Feb;59(2):555-66. doi: 10.1002/hep.26712. Epub 2013 Dec 20. Hepatology. 2014. PMID: 24038073 Free PMC article.
-
E2F1-mediated KDM4A-AS1 up-regulation promotes EMT of hepatocellular carcinoma cells by recruiting ILF3 to stabilize AURKA mRNA.Cancer Gene Ther. 2023 Jul;30(7):1007-1017. doi: 10.1038/s41417-023-00607-0. Epub 2023 Mar 27. Cancer Gene Ther. 2023. PMID: 36973424 Free PMC article.
-
Aurora A kinase (AURKA) in normal and pathological cell division.Cell Mol Life Sci. 2013 Feb;70(4):661-87. doi: 10.1007/s00018-012-1073-7. Epub 2012 Aug 3. Cell Mol Life Sci. 2013. PMID: 22864622 Free PMC article. Review.
Cited by
-
Control of centrin stability by Aurora A.PLoS One. 2011;6(6):e21291. doi: 10.1371/journal.pone.0021291. Epub 2011 Jun 23. PLoS One. 2011. PMID: 21731694 Free PMC article.
-
Long noncoding RNAs and tumorigenesis: genetic associations, molecular mechanisms, and therapeutic strategies.Tumour Biol. 2016 Jan;37(1):163-75. doi: 10.1007/s13277-015-4445-4. Epub 2015 Nov 19. Tumour Biol. 2016. PMID: 26586396 Review.
-
E2F and microRNA regulation of angiogenesis.Am J Cardiovasc Dis. 2011;1(2):110-118. Am J Cardiovasc Dis. 2011. PMID: 22200034 Free PMC article.
-
MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secretory phenotype through sirtuin 4/SIRT4.Aging (Albany NY). 2016 Mar;8(3):484-505. doi: 10.18632/aging.100905. Aging (Albany NY). 2016. PMID: 26959556 Free PMC article.
-
OncomiR-17-5p: alarm signal in cancer?Oncotarget. 2017 Jul 18;8(41):71206-71222. doi: 10.18632/oncotarget.19331. eCollection 2017 Sep 19. Oncotarget. 2017. PMID: 29050357 Free PMC article. Review.
References
-
- Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, Plowman GD. A homologue of Drosophila Aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. - DOI - PMC - PubMed
-
- Rojanala S, Han H, Munoz RM, Browne W, Nagle R, Von Hoff DD, Bearss DJ. The mitotic serine threonine kinase, Aurora-2, is a potential target for drug development in human pancreatic cancer. Mol Cancer Ther. 2004;3:451–457. - PubMed
-
- Tanner MM, Grenman S, Koul A, Johannsson O, Meltzer P, Pejovic T, Borg A, Isola JJ. Frequent amplification of chromosomal region 20q12–q13 in ovarian cancer. Clin Cancer Res. 2000;6:1833–1839. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

