Neocortical axon arbors trade-off material and conduction delay conservation
- PMID: 20300651
- PMCID: PMC2837396
- DOI: 10.1371/journal.pcbi.1000711
Neocortical axon arbors trade-off material and conduction delay conservation
Abstract
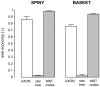
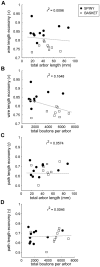
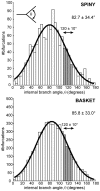
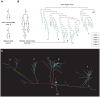
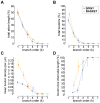
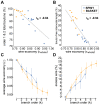
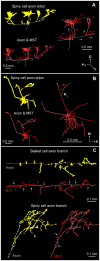
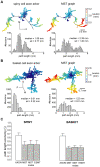
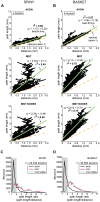
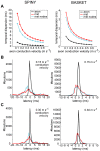
The brain contains a complex network of axons rapidly communicating information between billions of synaptically connected neurons. The morphology of individual axons, therefore, defines the course of information flow within the brain. More than a century ago, Ramón y Cajal proposed that conservation laws to save material (wire) length and limit conduction delay regulate the design of individual axon arbors in cerebral cortex. Yet the spatial and temporal communication costs of single neocortical axons remain undefined. Here, using reconstructions of in vivo labelled excitatory spiny cell and inhibitory basket cell intracortical axons combined with a variety of graph optimization algorithms, we empirically investigated Cajal's conservation laws in cerebral cortex for whole three-dimensional (3D) axon arbors, to our knowledge the first study of its kind. We found intracortical axons were significantly longer than optimal. The temporal cost of cortical axons was also suboptimal though far superior to wire-minimized arbors. We discovered that cortical axon branching appears to promote a low temporal dispersion of axonal latencies and a tight relationship between cortical distance and axonal latency. In addition, inhibitory basket cell axonal latencies may occur within a much narrower temporal window than excitatory spiny cell axons, which may help boost signal detection. Thus, to optimize neuronal network communication we find that a modest excess of axonal wire is traded-off to enhance arbor temporal economy and precision. Our results offer insight into the principles of brain organization and communication in and development of grey matter, where temporal precision is a crucial prerequisite for coincidence detection, synchronization and rapid network oscillations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


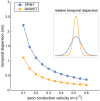

Similar articles
-
Properties of action-potential initiation in neocortical pyramidal cells: evidence from whole cell axon recordings.J Neurophysiol. 2007 Jan;97(1):746-60. doi: 10.1152/jn.00922.2006. Epub 2006 Nov 8. J Neurophysiol. 2007. PMID: 17093120
-
Neural arbors are Pareto optimal.Proc Biol Sci. 2019 May 15;286(1902):20182727. doi: 10.1098/rspb.2018.2727. Proc Biol Sci. 2019. PMID: 31039719 Free PMC article.
-
Communication and wiring in the cortical connectome.Front Neuroanat. 2012 Oct 16;6:42. doi: 10.3389/fnana.2012.00042. eCollection 2012. Front Neuroanat. 2012. PMID: 23087619 Free PMC article.
-
Neuronal circuits of the neocortex.Annu Rev Neurosci. 2004;27:419-51. doi: 10.1146/annurev.neuro.27.070203.144152. Annu Rev Neurosci. 2004. PMID: 15217339 Review.
-
Self-avoidance and tiling: Mechanisms of dendrite and axon spacing.Cold Spring Harb Perspect Biol. 2010 Sep;2(9):a001750. doi: 10.1101/cshperspect.a001750. Epub 2010 Jun 23. Cold Spring Harb Perspect Biol. 2010. PMID: 20573716 Free PMC article. Review.
Cited by
-
Versatile morphometric analysis and visualization of the three-dimensional structure of neurons.Neuroinformatics. 2013 Oct;11(4):393-403. doi: 10.1007/s12021-013-9188-z. Neuroinformatics. 2013. PMID: 23765606
-
Trade-off between multiple constraints enables simultaneous formation of modules and hubs in neural systems.PLoS Comput Biol. 2013;9(3):e1002937. doi: 10.1371/journal.pcbi.1002937. Epub 2013 Mar 7. PLoS Comput Biol. 2013. PMID: 23505352 Free PMC article.
-
EM connectomics reveals axonal target variation in a sequence-generating network.Elife. 2017 Mar 27;6:e24364. doi: 10.7554/eLife.24364. Elife. 2017. PMID: 28346140 Free PMC article.
-
Unsupervised classification of brain-wide axons reveals the presubiculum neuronal projection blueprint.Nat Commun. 2024 Feb 20;15(1):1555. doi: 10.1038/s41467-024-45741-x. Nat Commun. 2024. PMID: 38378961 Free PMC article.
-
An Optimized Structure-Function Design Principle Underlies Efficient Signaling Dynamics in Neurons.Sci Rep. 2018 Jul 11;8(1):10460. doi: 10.1038/s41598-018-28527-2. Sci Rep. 2018. PMID: 29992977 Free PMC article.
References
-
- Foh E, Haug H, König M, Rast A. Quantitative bestimmung zum feineren aufbau der sehrinde der katze, zugleich ein methodischer beitrag zur messung des neuropils. Microsc Acta. 1973;75:148–168. - PubMed
-
- Braitenberg V, Schüz A. Berlin, Germany: Springer-Verlag; 1991. Anatomy of the Cortex: Statistics and Geometry.
-
- Peters A, Payne BR. Numerical relationships between geniculocortical afferents and pyramidal cell modules in cat primary visual cortex. Cereb Cortex. 1993;3:69–78. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

