Ontogeny of stromal organizer cells during lymph node development
- PMID: 20237296
- PMCID: PMC2862734
- DOI: 10.4049/jimmunol.0903113
Ontogeny of stromal organizer cells during lymph node development
Abstract
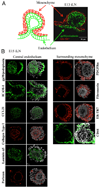
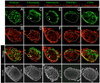
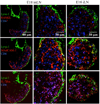
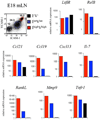
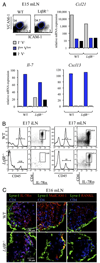
The development of secondary lymphoid organs, such as lymph nodes (LNs), in the embryo results from the reciprocal action between lymphoid tissue inducer (LTi) cells and stromal cells. However, the initial events inducing LN anlagen formation before the LTi stromal cells cross-talk interactions take place are not fully elucidated. In this study, we show that the inguinal LN anlagen in mouse embryos developed from mesenchymal cells surrounding the lymph sacs, spherical structures of endothelial cells that bud from veins. Using inguinal and mesenteric LNs (mLNs), we provide evidence supporting a two-step maturation model for stromal cells: first, ICAM-1(-)VCAM-1(-) mesenchymal precursor cells become ICAM-1(int)VCAM-1(int) cells, in a process independent of LTi cells and lymphotoxin beta receptor (LTbetaR) signaling. The second step involves the maturation of ICAM-1(int)VCAM-1(int) cells to ICAM-1(high)VCAM-1(high) mucosal addressin cell adhesion molecule-1(+) organizer cells and depends on both LTi cells and LTbetaR. Addition of alphaLTbetaR agonist to LN organ cultures was sufficient to induce ICAM-1(int)VCAM-1(int) cells to mature. In LtbetaR(-/-) embryos, both inguinal and mLN stromal cells showed a block at the ICAM-1(int)VCAM-1(int) stage, and, contrary to inguinal LNs, mLNs persist longer and contained LTi cells, which correlated with the sustained gene expression of Il-7, Cxcl13, and, to a lesser degree, Ccl21. Taken together, these results highlight the importance of the signals and cellular interactions that induce the maturation of stromal cells and ultimately lead to the formation of lymphoid tissues.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



Similar articles
-
LTbetaR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen.J Immunol. 2009 May 1;182(9):5439-45. doi: 10.4049/jimmunol.0801165. J Immunol. 2009. PMID: 19380791 Free PMC article.
-
Lymphotoxin a-dependent and -independent signals regulate stromal organizer cell homeostasis during lymph node organogenesis.Blood. 2007 Sep 15;110(6):1950-9. doi: 10.1182/blood-2007-01-070003. Epub 2007 May 25. Blood. 2007. PMID: 17526859
-
Kit ligand and Il7 differentially regulate Peyer's patch and lymph node development.J Immunol. 2010 Sep 15;185(6):3514-9. doi: 10.4049/jimmunol.1000665. Epub 2010 Aug 13. J Immunol. 2010. PMID: 20709954
-
A Fresh View on Lymph Node Organogenesis.Trends Immunol. 2018 Oct;39(10):775-787. doi: 10.1016/j.it.2018.08.003. Epub 2018 Aug 24. Trends Immunol. 2018. PMID: 30150089 Review.
-
Lymphoid Tissue inducer (LTi) cell ontogeny and functioning in embryo and adult.Biomed J. 2021 Apr;44(2):123-132. doi: 10.1016/j.bj.2020.12.003. Epub 2020 Dec 10. Biomed J. 2021. PMID: 33849806 Free PMC article. Review.
Cited by
-
Age-Associated Changes to Lymph Node Fibroblastic Reticular Cells.Front Aging. 2022 Jan 25;3:838943. doi: 10.3389/fragi.2022.838943. eCollection 2022. Front Aging. 2022. PMID: 35821826 Free PMC article.
-
KDM6B drives epigenetic reprogramming associated with lymphoid stromal cell early commitment and immune properties.Sci Adv. 2023 Dec;9(48):eadh2708. doi: 10.1126/sciadv.adh2708. Epub 2023 Nov 29. Sci Adv. 2023. PMID: 38019914 Free PMC article.
-
The mesenchymal context in inflammation, immunity and cancer.Nat Immunol. 2020 Sep;21(9):974-982. doi: 10.1038/s41590-020-0741-2. Epub 2020 Aug 3. Nat Immunol. 2020. PMID: 32747813 Review.
-
Organogenesis of Ileal Peyer's Patches Is Initiated Prenatally and Accelerated Postnatally With Comprehensive Proliferation of B Cells in Pigs.Front Immunol. 2020 Dec 4;11:604674. doi: 10.3389/fimmu.2020.604674. eCollection 2020. Front Immunol. 2020. PMID: 33424851 Free PMC article.
-
Rapid Generation of hPSC-Derived High Endothelial Venule Organoids with In Vivo Ectopic Lymphoid Tissue Capabilities.Adv Mater. 2024 Apr;36(15):e2308760. doi: 10.1002/adma.202308760. Epub 2024 Feb 11. Adv Mater. 2024. PMID: 38306610
References
-
- Mebius RE. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 2003;3:292–303. - PubMed
-
- Sabin F. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic in the pig. Am. J. Anat. 1902;1:367–389.
-
- Sabin F. The lymphatic system in human embryos, with a consideration of the morphology of the system as a whole. Am. J. Anat. 1909;1:43–91.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

