Macropinocytotic uptake and infection of human epithelial cells with species B2 adenovirus type 35
- PMID: 20237079
- PMCID: PMC2863792
- DOI: 10.1128/JVI.02494-09
Macropinocytotic uptake and infection of human epithelial cells with species B2 adenovirus type 35
Abstract
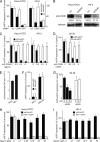
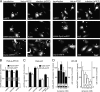
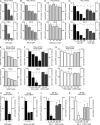
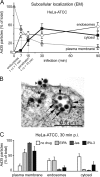
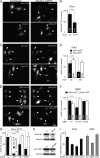
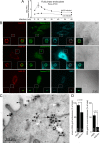
Human adenovirus serotype 35 (HAdV-35; here referred to as Ad35) causes kidney and urinary tract infections and infects respiratory organs of immunocompromised individuals. Unlike other adenoviruses, Ad35 has a low seroprevalence, which makes Ad35-based vectors promising candidates for gene therapy. Ad35 utilizes CD46 and integrins as receptors for infection of epithelial and hematopoietic cells. Here we show that infectious entry of Ad35 into HeLa cells, human kidney HK-2 cells, and normal human lung fibroblasts strongly depended on CD46 and integrins but not heparan sulfate and variably required the large GTPase dynamin. Ad35 infections were independent of expression of the carboxy-terminal domain of AP180, which effectively blocks clathrin-mediated uptake. Ad35 infections were inhibited by small chemicals against serine/threonine kinase Pak1 (p21-activated kinase), protein kinase C (PKC), sodium-proton exchangers, actin, and acidic organelles. Remarkably, the F-actin inhibitor jasplakinolide, the Pak1 inhibitor IPA-3, or the sodium-proton exchange inhibitor 5-(N-ethyl-N-isopropyl) amiloride (EIPA) blocked endocytic uptake of Ad35. Dominant-negative proteins or small interfering RNAs against factors driving macropinocytosis, including the small GTPase Rac1, Pak1, or the Pak1 effector C-terminal binding protein 1 (CtBP1), potently inhibited Ad35 infection. Confocal laser scanning microscopy, electron microscopy, and live cell imaging showed that Ad35 colocalized with fluid-phase markers in large endocytic structures that were positive for CD46, alphanu integrins, and also CtBP1. Our results extend earlier observations with HAdV-3 (Ad3) and establish macropinocytosis as an infectious pathway for species B human adenoviruses in epithelial and hematopoietic cells.
Figures

Similar articles
-
Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3.EMBO J. 2008 Apr 9;27(7):956-69. doi: 10.1038/emboj.2008.38. Epub 2008 Mar 6. EMBO J. 2008. PMID: 18323776 Free PMC article.
-
Entry of Epidemic Keratoconjunctivitis-Associated Human Adenovirus Type 37 in Human Corneal Epithelial Cells.Invest Ophthalmol Vis Sci. 2020 Aug 3;61(10):50. doi: 10.1167/iovs.61.10.50. Invest Ophthalmol Vis Sci. 2020. PMID: 32852546 Free PMC article.
-
Measles Virus Enters Breast and Colon Cancer Cell Lines through a PVRL4-Mediated Macropinocytosis Pathway.J Virol. 2017 Apr 28;91(10):e02191-16. doi: 10.1128/JVI.02191-16. Print 2017 May 15. J Virol. 2017. PMID: 28250131 Free PMC article.
-
Adenovirus endocytosis.J Gene Med. 2004 Feb;6 Suppl 1:S152-63. doi: 10.1002/jgm.553. J Gene Med. 2004. PMID: 14978758 Review.
-
Adenovirus endocytosis.J Gene Med. 2003 Jun;5(6):451-62. doi: 10.1002/jgm.409. J Gene Med. 2003. PMID: 12797110 Review.
Cited by
-
Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys.J Virol. 2012 Sep;86(18):9590-8. doi: 10.1128/JVI.00740-12. Epub 2012 Jul 11. J Virol. 2012. PMID: 22787208 Free PMC article.
-
Potential Diagnostic and Prognostic Biomarkers for Adenovirus Respiratory Infection in Children and Young Adults.Viruses. 2021 Sep 21;13(9):1885. doi: 10.3390/v13091885. Viruses. 2021. PMID: 34578465 Free PMC article. Review.
-
A comparative review of viral entry and attachment during large and giant dsDNA virus infections.Arch Virol. 2017 Dec;162(12):3567-3585. doi: 10.1007/s00705-017-3497-8. Epub 2017 Sep 2. Arch Virol. 2017. PMID: 28866775 Free PMC article. Review.
-
From Cell Entry to Engraftment of Exogenous Mitochondria.Int J Mol Sci. 2020 Jul 15;21(14):4995. doi: 10.3390/ijms21144995. Int J Mol Sci. 2020. PMID: 32679802 Free PMC article. Review.
-
Vaccinia extracellular virions enter cells by macropinocytosis and acid-activated membrane rupture.EMBO J. 2011 Jul 26;30(17):3647-61. doi: 10.1038/emboj.2011.245. EMBO J. 2011. PMID: 21792173 Free PMC article.
References
-
- Albert, M. L. 2004. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat. Rev. Immunol. 4:223-231. - PubMed
-
- Albinsson, B., and A. H. Kidd. 1999. Adenovirus type 41 lacks an RGD alpha(v)-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res. 64:125-136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

