Analysis of drug-protein binding by ultrafast affinity chromatography using immobilized human serum albumin
- PMID: 20227701
- PMCID: PMC2849903
- DOI: 10.1016/j.chroma.2010.02.026
Analysis of drug-protein binding by ultrafast affinity chromatography using immobilized human serum albumin
Abstract
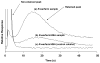
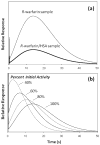
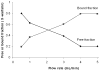
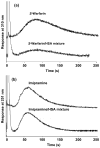
Human serum albumin (HSA) was explored for use as a stationary phase and ligand in affinity microcolumns for the ultrafast extraction of free drug fractions and the use of this information for the analysis of drug-protein binding. Warfarin, imipramine, and ibuprofen were used as model analytes in this study. It was found that greater than 95% extraction of all these drugs could be achieved in as little as 250 ms on HSA microcolumns. The retained drug fraction was then eluted from the same column under isocratic conditions, giving elution in less than 40 s when working at 4.5 mL/min. The chromatographic behavior of this system gave a good fit with that predicted by computer simulations based on a reversible, saturable model for the binding of an injected drug with immobilized HSA. The free fractions measured by this method were found to be comparable to those determined by ultrafiltration, and equilibrium constants estimated by this approach gave good agreement with literature values. Advantages of this method include its speed and the relatively low cost of microcolumns that contain HSA. The ability of HSA to bind many types of drugs also creates the possibility of using the same affinity microcolumn to study and measure the free fractions for a variety of pharmaceutical agents. These properties make this technique appealing for use in drug-binding studies and in the high-throughput screening of new drug candidates.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures
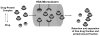
Similar articles
-
Analysis of free drug fractions in serum by ultrafast affinity extraction and two-dimensional affinity chromatography using α1-acid glycoprotein microcolumns.J Chromatogr A. 2016 Feb 5;1432:49-57. doi: 10.1016/j.chroma.2015.12.084. Epub 2016 Jan 4. J Chromatogr A. 2016. PMID: 26797422 Free PMC article.
-
Evaluation of affinity microcolumns containing human serum albumin for rapid analysis of drug-protein binding.J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Jun 15;878(20):1707-13. doi: 10.1016/j.jchromb.2010.04.028. Epub 2010 Apr 24. J Chromatogr B Analyt Technol Biomed Life Sci. 2010. PMID: 20462808 Free PMC article.
-
Analysis of free drug fractions by ultrafast affinity extraction: interactions of sulfonylurea drugs with normal or glycated human serum albumin.J Chromatogr A. 2014 Dec 5;1371:82-9. doi: 10.1016/j.chroma.2014.10.092. Epub 2014 Oct 31. J Chromatogr A. 2014. PMID: 25456590 Free PMC article.
-
Analysis of biomolecular interactions using affinity microcolumns: a review.J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Oct 1;968:49-63. doi: 10.1016/j.jchromb.2014.01.026. Epub 2014 Jan 27. J Chromatogr B Analyt Technol Biomed Life Sci. 2014. PMID: 24572459 Free PMC article. Review.
-
High-performance affinity chromatography and immobilized serum albumin as probes for drug- and hormone-protein binding.J Chromatogr B Biomed Sci Appl. 2000 Feb 28;739(1):39-54. doi: 10.1016/s0378-4347(99)00445-4. J Chromatogr B Biomed Sci Appl. 2000. PMID: 10744312 Review.
Cited by
-
Natural Alpha-Glucosidase Inhibitors Rapid Fishing from Cyperus Rotundus Using Immobilized Enzyme Affinity Screening Combined with UHPLC-QTOF MS.Iran J Pharm Res. 2019 Summer;18(3):1508-1515. doi: 10.22037/ijpr.2019.1100753. Iran J Pharm Res. 2019. PMID: 32641959 Free PMC article.
-
Analytical methods for kinetic studies of biological interactions: A review.J Pharm Biomed Anal. 2015 Sep 10;113:163-80. doi: 10.1016/j.jpba.2015.01.042. Epub 2015 Jan 27. J Pharm Biomed Anal. 2015. PMID: 25700721 Free PMC article. Review.
-
Binding studies based on ultrafast affinity extraction and single- or two-column systems: Interactions of second- and third-generation sulfonylurea drugs with normal or glycated human serum albumin.J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Dec 1;1102-1103:8-16. doi: 10.1016/j.jchromb.2018.10.015. Epub 2018 Oct 17. J Chromatogr B Analyt Technol Biomed Life Sci. 2018. PMID: 30366211 Free PMC article.
-
Research in bioanalysis and separations at the University of Nebraska - Lincoln.Bioanalysis. 2011 May;3(10):1065-76. doi: 10.4155/bio.11.64. Bioanalysis. 2011. PMID: 21585300 Free PMC article.
-
Analysis of Hormone-Protein Binding in Solution by Ultrafast Affinity Extraction: Interactions of Testosterone with Human Serum Albumin and Sex Hormone Binding Globulin.Anal Chem. 2015 Nov 17;87(22):11187-94. doi: 10.1021/acs.analchem.5b03007. Epub 2015 Oct 27. Anal Chem. 2015. PMID: 26484387 Free PMC article.
References
-
- MacKichan JJ. J Clin Pharmacokin. 1984;9:32. - PubMed
-
- Gilman AG, Rall TW, Nies AS, Taylor P. The Pharmacological Basis of Therapeutics. Pergamon Press; New York: 1990.
-
- Herve F, Urien S, Albengres E, Duche JC, Tillement JP. Clin Pharmacokin. 1994;26:44. - PubMed
-
- Kwong TC. Clin Chim Acta. 1985;151:193. - PubMed
-
- Liu M, Nicholson JK, Lindon JC. Anal Comm. 1997;34:225.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

