Zebrafish chordin-like and chordin are functionally redundant in regulating patterning of the dorsoventral axis
- PMID: 20226780
- PMCID: PMC2862114
- DOI: 10.1016/j.ydbio.2010.03.001
Zebrafish chordin-like and chordin are functionally redundant in regulating patterning of the dorsoventral axis
Abstract
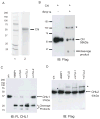
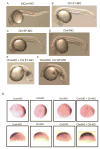
Chordin is the prototype of a group of cysteine-rich domain-containing proteins that bind and modulate signaling of various TGFbeta-like ligands. Chordin-like 1 and 2 (CHL1 and 2) are two members of this group that have been described in human, mouse, and chick. However, in vivo roles for CHL1 and 2 in early development are unknown due to lack of loss-of-function analysis. Here we identify and characterize zebrafish, Danio rerio, CHL (Chl). The chl gene is on a region of chromosome 21 syntenic with the area of murine chromosome 7 bearing the CHL2 gene. Inability to identify a separate zebrafish gene corresponding to the mammalian CHL1 gene suggests that Chl may serve roles in zebrafish distributed between CHL1 and CHL2 in other species. Chl is a maternal factor that is also zygotically expressed later in development and has spatiotemporal expression patterns that differ from but overlap those of zebrafish chordin (Chd), suggesting differences but also possible overlap in developmental roles of the two proteins. Chl, like Chd, dorsalizes embryos upon overexpression and is cleaved by BMP1, which antagonizes this activity. Loss-of-function experiments demonstrate that Chl serves as a BMP antagonist with functions that overlap and are redundant with those of Chd in forming the dorsoventral axis.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures







Similar articles
-
bmp1 and mini fin are functionally redundant in regulating formation of the zebrafish dorsoventral axis.Mech Dev. 2006 Jul;123(7):548-58. doi: 10.1016/j.mod.2006.05.004. Epub 2006 May 25. Mech Dev. 2006. PMID: 16824737
-
A beta1,4-galactosyltransferase is required for Bmp2-dependent patterning of the dorsoventral axis during zebrafish embryogenesis.Development. 2006 Jun;133(11):2233-41. doi: 10.1242/dev.02378. Epub 2006 May 3. Development. 2006. PMID: 16672343
-
Cleavage of the BMP-4 antagonist chordin by zebrafish tolloid.Science. 1997 Dec 12;278(5345):1937-40. doi: 10.1126/science.278.5345.1937. Science. 1997. PMID: 9395394
-
The Chordin Morphogenetic Pathway.Curr Top Dev Biol. 2016;116:231-45. doi: 10.1016/bs.ctdb.2015.10.003. Epub 2016 Jan 7. Curr Top Dev Biol. 2016. PMID: 26970622 Review.
-
Maternal and zygotic control of zebrafish dorsoventral axial patterning.Annu Rev Genet. 2011;45:357-77. doi: 10.1146/annurev-genet-110410-132517. Epub 2011 Sep 13. Annu Rev Genet. 2011. PMID: 21942367 Review.
Cited by
-
Expression and functional study of extracellular BMP antagonists during the morphogenesis of the digits and their associated connective tissues.PLoS One. 2013;8(4):e60423. doi: 10.1371/journal.pone.0060423. Epub 2013 Apr 3. PLoS One. 2013. PMID: 23573253 Free PMC article.
-
Comparative transcriptomics identifies genes differentially expressed in the intestine of a new fast-growing strain of common carp with higher unsaturated fatty acid content in muscle.PLoS One. 2018 Nov 5;13(11):e0206615. doi: 10.1371/journal.pone.0206615. eCollection 2018. PLoS One. 2018. PMID: 30395585 Free PMC article.
-
Distinct functions of alternatively spliced isoforms encoded by zebrafish mef2ca and mef2cb.Biochim Biophys Acta. 2014 Jul;1839(7):559-70. doi: 10.1016/j.bbagrm.2014.05.003. Epub 2014 May 17. Biochim Biophys Acta. 2014. PMID: 24844180 Free PMC article.
-
TGF-β Family Signaling in Early Vertebrate Development.Cold Spring Harb Perspect Biol. 2018 Jun 1;10(6):a033274. doi: 10.1101/cshperspect.a033274. Cold Spring Harb Perspect Biol. 2018. PMID: 28600394 Free PMC article. Review.
-
Agonists and Antagonists of TGF-β Family Ligands.Cold Spring Harb Perspect Biol. 2016 Aug 1;8(8):a021923. doi: 10.1101/cshperspect.a021923. Cold Spring Harb Perspect Biol. 2016. PMID: 27413100 Free PMC article. Review.
References
-
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–61. - PubMed
-
- Blader P, Rastegar S, Fischer N, Strahle U. Cleavage of the BMP-4 antagonist chordin by zebrafish tolloid. Science. 1997;278:1937–40. - PubMed
-
- Coffinier C, Tran U, Larrain J, De Robertis EM. Neuralin-1 is a novel Chordin-related molecule expressed in the mouse neural plate. Mech Dev. 2001;100:119–22. - PubMed
-
- Dal-Pra S, Furthauer M, Van-Celst J, Thisse B, Thisse C. Noggin1 and Follistatin-like2 function redundantly to Chordin to antagonize BMP activity. Dev Biol. 2006;298:514–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

