Sip1, the Drosophila orthologue of EBP50/NHERF1, functions with the sterile 20 family kinase Slik to regulate Moesin activity
- PMID: 20215404
- PMCID: PMC2844318
- DOI: 10.1242/jcs.059469
Sip1, the Drosophila orthologue of EBP50/NHERF1, functions with the sterile 20 family kinase Slik to regulate Moesin activity
Abstract
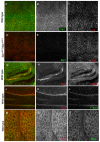
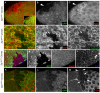
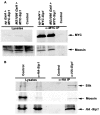
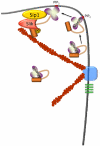
Organization of the plasma membrane in polarized epithelial cells is accomplished by the specific localization of transmembrane or membrane-associated proteins, which are often linked to cytoplasmic protein complexes, including the actin cytoskeleton. In this study, we identified Sip1 as a Drosophila orthologue of the ezrin-radixin-moesin (ERM) binding protein 50 (EBP50; also known as the Na(+)/H(+) exchanger regulatory factor NHERF1). In mammals, EBP50/NHERF1 is a scaffold protein required for the regulation of several transmembrane receptors and downstream signal transduction activity. In Drosophila, loss of Sip1 leads to a reduction in Slik kinase protein abundance, loss of Moesin phosphorylation and changes in epithelial structure, including mislocalization of E-cadherin and F-actin. Consistent with these findings, Moesin and Sip1 act synergistically in genetic-interaction experiments, and Sip1 protein abundance is dependent on Moesin. Co-immunoprecipitation experiments indicate that Sip1 forms a complex with both Moesin and Slik. Taken together, these data suggest that Sip1 promotes Slik-dependent phosphorylation of Moesin, and suggests a mechanism for the regulation of Moesin activity within the cell to maintain epithelial integrity.
Figures




Similar articles
-
Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia.Proc Natl Acad Sci U S A. 2004 Dec 21;101(51):17705-10. doi: 10.1073/pnas.0407974101. Epub 2004 Dec 10. Proc Natl Acad Sci U S A. 2004. PMID: 15591354 Free PMC article.
-
Foxj1 is required for apical localization of ezrin in airway epithelial cells.J Cell Sci. 2003 Dec 15;116(Pt 24):4935-45. doi: 10.1242/jcs.00830. J Cell Sci. 2003. PMID: 14625387
-
Regulation of Catalytic and Non-catalytic Functions of the Drosophila Ste20 Kinase Slik by Activation Segment Phosphorylation.J Biol Chem. 2015 Aug 21;290(34):20960-20971. doi: 10.1074/jbc.M115.645952. Epub 2015 Jul 13. J Biol Chem. 2015. PMID: 26170449 Free PMC article.
-
Roles of NHERF1/EBP50 in cancer.Curr Mol Med. 2008 Sep;8(6):459-68. doi: 10.2174/156652408785748031. Curr Mol Med. 2008. PMID: 18781953 Review.
-
ERM-Merlin and EBP50 protein families in plasma membrane organization and function.Annu Rev Cell Dev Biol. 2000;16:113-43. doi: 10.1146/annurev.cellbio.16.1.113. Annu Rev Cell Dev Biol. 2000. PMID: 11031232 Review.
Cited by
-
Focal defects in single-celled tubes mutant for Cerebral cavernous malformation 3, GCKIII, or NSF2.Dev Cell. 2013 Jun 10;25(5):507-19. doi: 10.1016/j.devcel.2013.05.002. Dev Cell. 2013. PMID: 23763949 Free PMC article.
-
NRFL-1, the C. elegans NHERF orthologue, interacts with amino acid transporter 6 (AAT-6) for age-dependent maintenance of AAT-6 on the membrane.PLoS One. 2012;7(8):e43050. doi: 10.1371/journal.pone.0043050. Epub 2012 Aug 15. PLoS One. 2012. PMID: 22916205 Free PMC article.
-
Ezrin-mediated apical integrity is required for intestinal homeostasis.Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):11924-9. doi: 10.1073/pnas.1103418108. Epub 2011 Jul 5. Proc Natl Acad Sci U S A. 2011. PMID: 21730140 Free PMC article.
-
Slik and the receptor tyrosine kinase Breathless mediate localized activation of Moesin in terminal tracheal cells.PLoS One. 2014 Jul 25;9(7):e103323. doi: 10.1371/journal.pone.0103323. eCollection 2014. PLoS One. 2014. PMID: 25061859 Free PMC article.
-
ERM-1 Phosphorylation and NRFL-1 Redundantly Control Lumen Formation in the C. elegans Intestine.Front Cell Dev Biol. 2022 Feb 7;10:769862. doi: 10.3389/fcell.2022.769862. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35198555 Free PMC article.
References
-
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403-410 - PubMed
-
- Amieva M. R., Wilgenbus K. K., Furthmayr H. (1994). Radixin is a component of hepatocyte microvilli in situ. Exp. Cell Res. 210, 140-144 - PubMed
-
- Bateman J., Reddy R. S., Saito H., Van Vactor D. (2001). The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr. Biol. 11, 1317-1327 - PubMed
-
- Berryman M., Franck Z., Bretscher A. (1993). Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J. Cell Sci. 105, 1025-1043 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

