Responding to pandemic (H1N1) 2009 influenza: the role of oseltamivir
- PMID: 20215134
- PMCID: PMC2835510
- DOI: 10.1093/jac/dkq014
Responding to pandemic (H1N1) 2009 influenza: the role of oseltamivir
Abstract
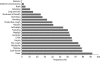
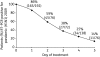
Pandemic (H1N1) 2009 influenza is affecting countries in all five continents, with most cases so far having been reported in North and South America and Europe, and children and young adults being the most susceptible age groups. To date, the clinical course of disease is typically mild, with low hospitalization and mortality rates. Pandemic (H1N1) 2009 is susceptible to oseltamivir and, although few clinical data are yet available, current information suggests that treatment with oseltamivir appears to be beneficial. Only isolated cases of resistance to the drug have been reported to date, in keeping with the low frequency observed in clinical studies involving patients infected with seasonal influenza viruses. Current health authority guidelines recommend the use of oseltamivir in infected adults and children who have or are at elevated risk for severe disease, including pregnant women; use during the pandemic in infants < 1 year has also been authorized in Europe and a number of other countries, including the USA and Canada. Before the onset of the current pandemic, F. Hoffmann-La Roche Ltd expanded annual production capacity for oseltamivir to 400 million treatment courses per year to meet anticipated demand. However, during an influenza pandemic, and despite increased production capabilities, resources are nonetheless likely to be initially in short supply. For this reason, Roche, in line with WHO recommendations, has advocated advance stockpiling of antivirals by governments as a pandemic preparedness measure. Between 2004 and December 2009, 350 million treatment courses were supplied to governments worldwide. Support for developing countries has been a priority. Roche has established a cluster of initiatives aimed at increasing access to Tamiflu for the world's developing economies, including, making donations to the WHO, establishing the Tamiflu Reserves Program (TRP) and sub-licensing and manufacturing contracts with local companies in Asia and Africa. Furthermore, Roche has published a document outlining how it would allocate limited supplies of Tamiflu during a pandemic, which are in line with WHO recommendations stating that 'resources should be used to provide the maximum possible health benefit'. Roche is also offering support such as reprocessing of expiring capsule stocks (in development) and shelf-life extension to support governments in the management of their stockpiles. Clinical studies, either sponsored by or supported by Roche, are in progress. These trials are designed to investigate the effectiveness of oseltamivir in patients infected with the pandemic virus in greater depth, and include high-dose studies, assessment of natural and drug-induced resistance, and response to treatment in high-risk populations such as young infants, immunocompromised patients and the severely ill.
Figures
Similar articles
-
Oseltamivir in seasonal influenza: cumulative experience in low- and high-risk patients.J Antimicrob Chemother. 2010 Apr;65 Suppl 2(Suppl 2):ii11-ii24. doi: 10.1093/jac/dkq012. J Antimicrob Chemother. 2010. PMID: 20215131 Free PMC article. Review.
-
Selection for resistance to oseltamivir in seasonal and pandemic H1N1 influenza and widespread co-circulation of the lineages.Int J Health Geogr. 2010 Feb 24;9:13. doi: 10.1186/1476-072X-9-13. Int J Health Geogr. 2010. PMID: 20181276 Free PMC article.
-
Oseltamivir in seasonal, pandemic, and avian influenza: a comprehensive review of 10-years clinical experience.Adv Ther. 2011 Nov;28(11):927-59. doi: 10.1007/s12325-011-0072-7. Epub 2011 Nov 1. Adv Ther. 2011. PMID: 22057727 Free PMC article. Review.
-
Five years of non-prescription oseltamivir: effects on resistance, immunization and stockpiling.J Antimicrob Chemother. 2012 Dec;67(12):2949-56. doi: 10.1093/jac/dks337. Epub 2012 Sep 4. J Antimicrob Chemother. 2012. PMID: 22949624 Free PMC article.
-
Oseltamivir-resistant influenza viruses circulating during the first year of the influenza A(H1N1) 2009 pandemic in the Asia-Pacific region, March 2009 to March 2010.Euro Surveill. 2011 Jan 20;16(3):19770. Euro Surveill. 2011. PMID: 21262183
Cited by
-
Clinical characteristics of 2009 pandemic influenza A (H1N1) infection in children and the performance of rapid antigen test.Korean J Pediatr. 2011 Oct;54(10):405-8. doi: 10.3345/kjp.2011.54.10.405. Epub 2011 Oct 31. Korean J Pediatr. 2011. PMID: 22232622 Free PMC article.
-
High levels and safety of oseltamivir carboxylate plasma concentrations after nasogastric administration in critically ill children in a pediatric intensive care unit.Antimicrob Agents Chemother. 2011 Jan;55(1):433-5. doi: 10.1128/AAC.00813-10. Epub 2010 Oct 11. Antimicrob Agents Chemother. 2011. PMID: 20937783 Free PMC article.
-
Detection of SARS-CoV-2 in wastewater as an earlier predictor of COVID-19 epidemic peaks in Venezuela.Sci Rep. 2024 Nov 8;14(1):27294. doi: 10.1038/s41598-024-78982-3. Sci Rep. 2024. PMID: 39516586 Free PMC article.
-
Simple and sensitive assay for quantification of oseltamivir and its active metabolite oseltamivir carboxylate in human plasma using high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry: improved applicability to pharmacokinetic study.J Pharm Biomed Anal. 2013 Jan;72:245-50. doi: 10.1016/j.jpba.2012.08.029. Epub 2012 Sep 5. J Pharm Biomed Anal. 2013. PMID: 23000242 Free PMC article.
-
Control Measures for Human Respiratory Viral Infection.Semin Respir Crit Care Med. 2016 Aug;37(4):631-9. doi: 10.1055/s-0036-1584792. Epub 2016 Aug 3. Semin Respir Crit Care Med. 2016. PMID: 27486741 Free PMC article. Review.
References
-
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. - PubMed
-
- WHO. Pandemic (H1N1) 2009 Update 76. http://www.who.int/csr/don/2009_11_27a/en/index.html. (10 December 2009, date last accessed)
-
- WHO. Changes in Reporting Requirements for Pandemic (H1N1) 2009 Virus Infection. http://www.who.int/csr/disease/swineflu/notes/ h1n1_surveillance_2009071.... (22 October 2009, date last accessed)
-
- WHO. Human infection with pandemic A (H1N1) 2009 influenza virus: clinical observations in hospitalized patients, Americas, July 2009—update. Wkly Epidemiol Rec. 2009;84:305–8. - PubMed
-
- CDC. 2009 pandemic influenza A (H1N1) virus infections—Chicago, Illinois, April-July 2009. MMWR Morb Mortal Wkly Rep. 2009;58:536–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

