Characterization of somatic cell nuclear reprogramming by oocytes in which a linker histone is required for pluripotency gene reactivation
- PMID: 20212135
- PMCID: PMC2851752
- DOI: 10.1073/pnas.1000599107
Characterization of somatic cell nuclear reprogramming by oocytes in which a linker histone is required for pluripotency gene reactivation
Abstract
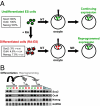
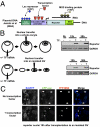
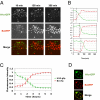
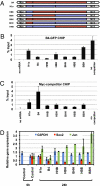
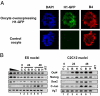
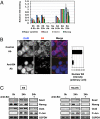
When transplanted into Xenopus oocytes, the nuclei of mammalian somatic cells are reprogrammed to express stem cell genes such as Oct4, Nanog, and Sox2. We now describe an experimental system in which the pluripotency genes Sox2 and Oct4 are repressed in retinoic acid-treated ES cells but are reprogrammed up to 100% within 24 h by injection of nuclei into the germinal vesicle (GV) of growing Xenopus oocytes. The isolation of GVs in nonaqueous medium allows the reprogramming of individual injected nuclei to be seen in real time. Analysis using fluorescence recovery after photobleaching shows that nuclear transfer is associated with an increase in linker histone mobility. A simultaneous loss of somatic H1 linker histone and incorporation of the oocyte-specific linker histone B4 precede transcriptional reprogramming. The loss of H1 is not required for gene reprogramming. We demonstrate both by antibody injection experiments and by dominant negative interference that the incorporation of B4 linker histone is required for pluripotency gene reactivation during nuclear reprogramming. We suggest that the binding of oocyte-specific B4 linker histone to chromatin is a key primary event in the reprogramming of somatic nuclei transplanted to amphibian oocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
A natural oocyte component required for the reprogramming of somatic cell nuclei.Cell Cycle. 2010 Jun 15;9(12):2261-2. doi: 10.4161/cc.9.12.12019. Epub 2010 Jun 15. Cell Cycle. 2010. PMID: 20519946 No abstract available.
Similar articles
-
Reprogramming of gene expression following nuclear transfer to the Xenopus oocyte.Biol Aujourdhui. 2011;205(2):105-10. doi: 10.1051/jbio/2011013. Epub 2011 Aug 11. Biol Aujourdhui. 2011. PMID: 21831341
-
Differential in vivo binding dynamics of somatic and oocyte-specific linker histones in oocytes and during ES cell nuclear transfer.Mol Biol Cell. 2005 Aug;16(8):3887-95. doi: 10.1091/mbc.e05-04-0350. Epub 2005 Jun 8. Mol Biol Cell. 2005. PMID: 15944219 Free PMC article.
-
Remodeling somatic nuclei in Xenopus laevis egg extracts: molecular mechanisms for the selective release of histones H1 and H1(0) from chromatin and the acquisition of transcriptional competence.EMBO J. 1996 Nov 1;15(21):5897-906. EMBO J. 1996. PMID: 8918467 Free PMC article.
-
Reprogramming of somatic cells and nuclei by Xenopus oocyte and egg extracts.Int J Dev Biol. 2016;60(7-8-9):289-296. doi: 10.1387/ijdb.160163at. Int J Dev Biol. 2016. PMID: 27251073 Review.
-
Epigenetic stability of repressed states involving the histone variant macroH2A revealed by nuclear transfer to Xenopus oocytes.Nucleus. 2011 Nov-Dec;2(6):533-9. doi: 10.4161/nucl.2.6.17799. Epub 2011 Nov 1. Nucleus. 2011. PMID: 22064467 Free PMC article. Review.
Cited by
-
Xenopus egg extract treatment reduced global DNA methylation of donor cells and enhanced somatic cell nuclear transfer embryo development in pigs.Biores Open Access. 2012 Apr;1(2):79-87. doi: 10.1089/biores.2012.0214. Biores Open Access. 2012. PMID: 23515109 Free PMC article.
-
Germline-specific H1 variants: the "sexy" linker histones.Chromosoma. 2016 Mar;125(1):1-13. doi: 10.1007/s00412-015-0517-x. Epub 2015 Apr 29. Chromosoma. 2016. PMID: 25921218 Review.
-
Non-viral expression of mouse Oct4, Sox2, and Klf4 transcription factors efficiently reprograms tadpole muscle fibers in vivo.J Biol Chem. 2012 Mar 2;287(10):7427-35. doi: 10.1074/jbc.M111.324368. Epub 2012 Jan 9. J Biol Chem. 2012. PMID: 22232554 Free PMC article.
-
Reactivation of the inactive X chromosome in development and reprogramming.Cell Mol Life Sci. 2013 Jul;70(14):2443-61. doi: 10.1007/s00018-012-1174-3. Epub 2012 Sep 30. Cell Mol Life Sci. 2013. PMID: 23052214 Free PMC article. Review.
-
Dedifferentiation of human uterine polyp stem cells into embryo-like cells during inducing pluripotency.Int J Biol Sci. 2018 Sep 7;14(11):1586-1598. doi: 10.7150/ijbs.23401. eCollection 2018. Int J Biol Sci. 2018. PMID: 30263010 Free PMC article.
References
-
- Gurdon JB. The developmental capacity of nuclei taken from differentiating endoderm cells of Xenopus laevis. J Embryol Exp Morphol. 1960;8:505–526. - PubMed
-
- Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

