Single-chain HLA-A2 MHC trimers that incorporate an immundominant peptide elicit protective T cell immunity against lethal West Nile virus infection
- PMID: 20212098
- PMCID: PMC3628725
- DOI: 10.4049/jimmunol.0903955
Single-chain HLA-A2 MHC trimers that incorporate an immundominant peptide elicit protective T cell immunity against lethal West Nile virus infection
Abstract
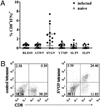
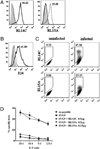
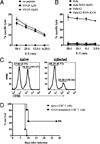
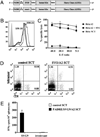
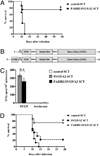
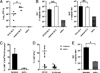
The generation of a robust CD8(+) T cell response is an ongoing challenge for the development of DNA vaccines. One problem encountered with classical DNA plasmid immunization is that peptides produced are noncovalently and transiently associated with MHC class I molecules and thus may not durably stimulate CD8(+) T cell responses. To address this and enhance the expression and presentation of the antigenic peptide/MHC complexes, we generated single-chain trimers (SCTs) composed of a single polypeptide chain with a linear composition of antigenic peptide, beta(2)-microglobulin, and H chain connected by flexible linkers. In this study, we test whether the preassembled nature of the SCT makes them effective for eliciting protective CD8(+) T cell responses against pathogens. A DNA plasmid was constructed encoding an SCT incorporating the human MHC class I molecule HLA-A2 and the immunodominant peptide SVG9 derived from the envelope protein of West Nile virus (WNV). HLA-A2 transgenic mice vaccinated with the DNA encoding the SVG9/HLA-A2 SCT generated a robust epitope-specific CD8(+) T cell response and showed enhanced survival rate and lower viral burden in the brain after lethal WNV challenge. Inclusion of a CD4(+) Th cell epitope within the SCT did not increase the frequency of SVG9-specific CD8(+) T cells, but did enhance protection against WNV challenge. Overall, these findings demonstrate that the SCT platform can induce protective CD8(+) T cell responses against lethal virus infection and may be paired with immunogens that elicit robust neutralizing Ab responses to generate vaccines that optimally activate all facets of adaptive immunity.
Figures

Similar articles
-
Single chain MHC I trimer-based DNA vaccines for protection against Listeria monocytogenes infection.Vaccine. 2012 Mar 9;30(12):2178-86. doi: 10.1016/j.vaccine.2012.01.012. Epub 2012 Jan 26. Vaccine. 2012. PMID: 22285270 Free PMC article.
-
West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection.J Immunol. 2008 Dec 15;181(12):8568-75. doi: 10.4049/jimmunol.181.12.8568. J Immunol. 2008. PMID: 19050276 Free PMC article.
-
Characterization of HLA-A2-restricted HPV-16 E7-specific CD8(+) T-cell immune responses induced by DNA vaccines in HLA-A2 transgenic mice.Gene Ther. 2006 Jan;13(1):67-77. doi: 10.1038/sj.gt.3302607. Gene Ther. 2006. PMID: 16107858 Free PMC article.
-
Mapping and analysis of West Nile virus-specific monoclonal antibodies: prospects for vaccine development.Expert Rev Vaccines. 2007 Apr;6(2):183-91. doi: 10.1586/14760584.6.2.183. Expert Rev Vaccines. 2007. PMID: 17408368 Review.
-
The molecular basis of antibody protection against West Nile virus.Curr Top Microbiol Immunol. 2008;317:125-53. doi: 10.1007/978-3-540-72146-8_5. Curr Top Microbiol Immunol. 2008. PMID: 17990792 Review.
Cited by
-
West Nile virus vaccines - current situation and future directions.Hum Vaccin Immunother. 2019;15(10):2337-2342. doi: 10.1080/21645515.2019.1621149. Epub 2019 Jul 10. Hum Vaccin Immunother. 2019. PMID: 31116691 Free PMC article. Review.
-
Optimized polyepitope neoantigen DNA vaccines elicit neoantigen-specific immune responses in preclinical models and in clinical translation.Genome Med. 2021 Apr 21;13(1):56. doi: 10.1186/s13073-021-00872-4. Genome Med. 2021. PMID: 33879241 Free PMC article.
-
Of Mice and Men: Protective and Pathogenic Immune Responses to West Nile virus Infection.Curr Trop Med Rep. 2015 Mar 1;2(1):41-48. doi: 10.1007/s40475-015-0040-4. Curr Trop Med Rep. 2015. PMID: 26120511 Free PMC article.
-
Potential targets for pancreatic cancer immunotherapeutics.Immunotherapy. 2011 Apr;3(4):517-37. doi: 10.2217/imt.11.10. Immunotherapy. 2011. PMID: 21463193 Free PMC article. Review.
-
Single chain MHC I trimer-based DNA vaccines for protection against Listeria monocytogenes infection.Vaccine. 2012 Mar 9;30(12):2178-86. doi: 10.1016/j.vaccine.2012.01.012. Epub 2012 Jan 26. Vaccine. 2012. PMID: 22285270 Free PMC article.
References
-
- Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu. Rev. Immunol. 2006;24:519–540. - PubMed
-
- Deng Y, Yewdell JW, Eisenlohr LC, Bennink JR. MHC affinity, peptide liberation, T cell repertoire, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by antiviral CTL. J. Immunol. 1997;158:1507–1515. - PubMed
-
- Yu YY, Netuschil N, Lybarger L, Connolly JM, Hansen TH. Cutting edge: single-chain trimers of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J. Immunol. 2002;168:3145–3149. - PubMed
-
- Greten TF, Korangy F, Neumann G, Wedemeyer H, Schlote K, Heller A, Scheffer S, Pardoll DM, Garbe AI, Schneck JP, Manns MP. Peptide-beta2-microglobulin-MHC fusion molecules bind antigen-specific T cells and can be used for multivalent MHC-Ig complexes. J. Immunol. Methods. 2002;271:125–135. - PubMed
-
- Choudhuri K, Wiseman D, Brown MH, Gould K, van der Merwe PA. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005;436:578–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

