The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens
- PMID: 20207024
- PMCID: PMC2891948
- DOI: 10.1016/j.tins.2010.02.002
The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens
Abstract
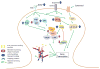
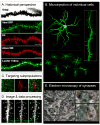
Addictive drugs cause persistent restructuring of several neuronal cell types in the limbic regions of brain thought to be responsible for long-term behavioral plasticity driving addiction. Although these structural changes are well documented in nucleus accumbens medium spiny neurons, little is known regarding the underlying molecular mechanisms. Additionally, it remains unclear whether structural plasticity and its synaptic concomitants drive addictive behaviors or whether they reflect homeostatic compensations to the drug not related to addiction per se. Here, we discuss recent paradoxical data, which either support or oppose the hypothesis that drug-induced changes in dendritic spines drive addictive behavior. We define areas where future investigation can provide a more detailed picture of drug-induced synaptic reorganization, including ultrastructural, electrophysiological and behavioral studies.
Figures

Similar articles
-
Structural plasticity associated with exposure to drugs of abuse.Neuropharmacology. 2004;47 Suppl 1:33-46. doi: 10.1016/j.neuropharm.2004.06.025. Neuropharmacology. 2004. PMID: 15464124 Review.
-
A novel mouse brain slice preparation of the hippocampo-accumbens pathway.J Neurosci Methods. 2004 Aug 15;137(1):49-60. doi: 10.1016/j.jneumeth.2004.02.001. J Neurosci Methods. 2004. PMID: 15196826
-
Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways.Mol Interv. 2002 Jun;2(3):146-57. doi: 10.1124/mi.2.3.146. Mol Interv. 2002. PMID: 14993375 Review. No abstract available.
-
Normalizing drug-induced neuronal plasticity in nucleus accumbens weakens enduring drug-seeking behavior.Neuropsychopharmacology. 2010 Jan;35(1):352-3. doi: 10.1038/npp.2009.97. Neuropsychopharmacology. 2010. PMID: 20010718 Free PMC article. No abstract available.
-
Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling.Neuron. 2011 Feb 24;69(4):650-63. doi: 10.1016/j.neuron.2011.01.017. Neuron. 2011. PMID: 21338877 Free PMC article. Review.
Cited by
-
Exploring the Role of DARPP-32 in Addiction: A Review of the Current Limitations of Addiction Treatment Pathways and the Role of DARPP-32 to Improve Them.NeuroSci. 2022 Aug 25;3(3):494-509. doi: 10.3390/neurosci3030035. eCollection 2022 Sep. NeuroSci. 2022. PMID: 39483434 Free PMC article. Review.
-
Prolonged Consumption of Sucrose in a Binge-Like Manner, Alters the Morphology of Medium Spiny Neurons in the Nucleus Accumbens Shell.Front Behav Neurosci. 2016 Mar 23;10:54. doi: 10.3389/fnbeh.2016.00054. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27047355 Free PMC article.
-
Dopamine activates NF-κB and primes the NLRP3 inflammasome in primary human macrophages.Brain Behav Immun Health. 2020 Feb;2:100030. doi: 10.1016/j.bbih.2019.100030. Epub 2019 Dec 31. Brain Behav Immun Health. 2020. PMID: 33665636 Free PMC article.
-
Reduced dopamine release in Dcc haploinsufficiency male mice abolishes the rewarding effects of cocaine but not those of morphine and ethanol.Psychopharmacology (Berl). 2023 Mar;240(3):637-646. doi: 10.1007/s00213-022-06288-1. Epub 2022 Dec 6. Psychopharmacology (Berl). 2023. PMID: 36471064 Free PMC article.
-
Beyond counts and shapes: studying pathology of dendritic spines in the context of the surrounding neuropil through serial section electron microscopy.Neuroscience. 2013 Oct 22;251:75-89. doi: 10.1016/j.neuroscience.2012.04.061. Epub 2012 May 1. Neuroscience. 2013. PMID: 22561733 Free PMC article. Review.
References
-
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. - PubMed
-
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20(6):1647–54. - PubMed

