Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation
- PMID: 20206617
- PMCID: PMC2862458
- DOI: 10.1016/j.ydbio.2010.02.033
Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation
Abstract
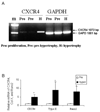
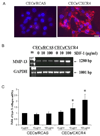
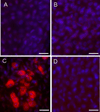
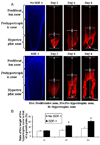
During endochondral bone formation, chondrocytes undergo differentiation toward hypertrophy before they are replaced by bone and bone marrow. In this study, we found that a G-protein coupled receptor CXCR4 is predominantly expressed in hypertrophic chondrocytes, while its ligand, chemokine stromal cell-derived factor 1 (SDF-1) is expressed in the bone marrow adjacent to hypertrophic chondrocytes. Thus, they are expressed in a complementary pattern in the chondro-osseous junction of the growth plate. Transfection of a CXCR4 cDNA into pre-hypertrophic chondrocytes results in a dose-dependent increase of hypertrophic markers including Runx2, Col X, and MMP-13 in response to SDF-1 treatment. In organ culture SDF-1 infiltrates cartilage and accelerates growth plate hypertrophy. Furthermore, a continuous infusion of SDF-1 into the rabbit proximal tibial physis results in early physeal closure, which is accompanied by a transient elevation of type X collagen expression. Blocking SDF-1/CXCR4 interaction suppresses the expression of Runx2. Thus, interaction of SDF-1 and CXCR4 is required for Runx2 expression. Interestingly, knocking down Runx2 gene expression results in a decrease of CXCR4 mRNA levels in hypertrophic chondrocytes. This suggests a positive feedback loop of stimulation of chondrocyte hypertrophy by SDF-1/CXCR4, which is mediated by Runx2.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Stromal cell-derived factor 1 regulates the actin organization of chondrocytes and chondrocyte hypertrophy.PLoS One. 2012;7(5):e37163. doi: 10.1371/journal.pone.0037163. Epub 2012 May 18. PLoS One. 2012. PMID: 22623989 Free PMC article.
-
Overexpression of transcription factor FoxA2 in the developing skeleton causes an enlargement of the cartilage hypertrophic zone, but it does not trigger ectopic differentiation in immature chondrocytes.Bone. 2022 Jul;160:116418. doi: 10.1016/j.bone.2022.116418. Epub 2022 Apr 6. Bone. 2022. PMID: 35398294 Free PMC article.
-
Continuous infusion of angiotensin II modulates hypertrophic differentiation and apoptosis of chondrocytes in cartilage formation in a fracture model mouse.Hypertens Res. 2015 Jun;38(6):382-93. doi: 10.1038/hr.2015.18. Epub 2015 Feb 19. Hypertens Res. 2015. PMID: 25693858
-
Smad-Runx interactions during chondrocyte maturation.J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S15-22. J Bone Joint Surg Am. 2001. PMID: 11263661 Review.
-
Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells.Eur Cell Mater. 2012 Jul 24;24:118-35; discussion 135. doi: 10.22203/ecm.v024a09. Eur Cell Mater. 2012. PMID: 22828990 Review.
Cited by
-
Inhibition of SDF-1α/CXCR4 Signalling in Subchondral Bone Attenuates Post-Traumatic Osteoarthritis.Int J Mol Sci. 2016 Jun 16;17(6):943. doi: 10.3390/ijms17060943. Int J Mol Sci. 2016. Retraction in: Int J Mol Sci. 2024 Jul 16;25(14):7757. doi: 10.3390/ijms25147757 PMID: 27322244 Free PMC article. Retracted.
-
The cytokine and chemokine expression profile of nucleus pulposus cells: implications for degeneration and regeneration of the intervertebral disc.Arthritis Res Ther. 2013;15(6):R213. doi: 10.1186/ar4408. Arthritis Res Ther. 2013. PMID: 24325988 Free PMC article.
-
Mitogen-activated protein kinase p38 induces HDAC4 degradation in hypertrophic chondrocytes.Biochim Biophys Acta. 2015 Feb;1853(2):370-376. doi: 10.1016/j.bbamcr.2014.11.003. Epub 2014 Nov 13. Biochim Biophys Acta. 2015. PMID: 25447540 Free PMC article.
-
Nutrition and degeneration of articular cartilage.Knee Surg Sports Traumatol Arthrosc. 2013 Aug;21(8):1751-62. doi: 10.1007/s00167-012-1977-7. Epub 2012 Apr 4. Knee Surg Sports Traumatol Arthrosc. 2013. PMID: 22476522 Free PMC article.
-
New findings in osteoarthritis pathogenesis: therapeutic implications.Ther Adv Chronic Dis. 2013 Jan;4(1):23-43. doi: 10.1177/2040622312462734. Ther Adv Chronic Dis. 2013. PMID: 23342245 Free PMC article.
References
-
- Cole AA, Luchene LJ, Linsenmayer TF, Schmid TM. The influence of bone and marrow on cartilage hypertrophy and degradation during 30-day serum-free culture of the embryonic chick tibia. Dev. Dyn. 1992;193:277–285. - PubMed
-
- Dong YF, Soung DY, Schwarz EM, O'Keefe RJ, Drissi H. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell. Physiol. 2006;208:77–86. - PubMed
-
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation.[see comment] Cell. 1997;89:747–754. - PubMed
-
- Fermas S, Gonnet F, Sutton A, Charnaux N, Mulloy B, Du Y, Baleux F, Daniel R. Sulfated oligosaccharides (heparin and fucoidan) binding and dimerization of stromal cell-derived factor-1 (SDF-1/CXCL 12) are coupled as evidenced by affinity CE-MS analysis. Glycobiology. 2008;18:1054–1064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG00811/AG/NIA NIH HHS/United States
- R03 AR052479/AR/NIAMS NIH HHS/United States
- R03 AR052479-01A1/AR/NIAMS NIH HHS/United States
- R01 AR059142/AR/NIAMS NIH HHS/United States
- R29 AG014399/AG/NIA NIH HHS/United States
- R01 AG014399/AG/NIA NIH HHS/United States
- P20 GM104937/GM/NIGMS NIH HHS/United States
- R03 AR052479-02/AR/NIAMS NIH HHS/United States
- P20 RR024484/RR/NCRR NIH HHS/United States
- AG14399/AG/NIA NIH HHS/United States
- Z01 AG000811/Intramural NIH HHS/United States
- AR052479/AR/NIAMS NIH HHS/United States
- P20RR024484/RR/NCRR NIH HHS/United States
- R03 AR052479-03/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

