Membrane targeted horseradish peroxidase as a marker for correlative fluorescence and electron microscopy studies
- PMID: 20204144
- PMCID: PMC2831632
- DOI: 10.3389/neuro.04.006.2010
Membrane targeted horseradish peroxidase as a marker for correlative fluorescence and electron microscopy studies
Abstract
Synaptic dynamics and reorganization are fundamental features of synaptic plasticity both during synaptic circuit development and in the mature CNS underlying learning, memory, and experience-dependent circuit rearrangements. Combining in vivo time-lapse fluorescence imaging and retrospective electron microscopic analysis provides a powerful technique to decipher the rules governing dynamics of neuronal structure and synaptic connections. Here we have generated a membrane-targeted horseradish peroxidase (mHRP) that allows identification of transfected cells without obscuring the intracellular ultrastructure or organelles and in particular allows identification of synaptic sites using electron microscopy. The expression of mHRP does not affect dendritic arbor growth or dynamics of transfected neurons. Co-expression of EGFP and mHRP was used to study neuronal morphology at both the light and electron microscopic levels. mHRP expression greatly facilitates 3D reconstruction based on serial EM sections. We expect this reagent will be valuable for studying the mechanisms that guide construction of neuronal networks.
Keywords: 3D reconstruction; Xenopus laevis; horseradish peroxidase; synapse; time-lapse imaging; ultrastructure.
Figures
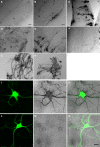
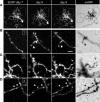
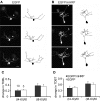
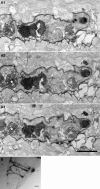


Similar articles
-
AMPA receptors regulate experience-dependent dendritic arbor growth in vivo.Proc Natl Acad Sci U S A. 2006 Aug 8;103(32):12127-31. doi: 10.1073/pnas.0602670103. Epub 2006 Aug 1. Proc Natl Acad Sci U S A. 2006. PMID: 16882725 Free PMC article.
-
Correlative microscopy.Arch Biochem Biophys. 2015 Sep 1;581:98-110. doi: 10.1016/j.abb.2015.05.017. Epub 2015 Jun 10. Arch Biochem Biophys. 2015. PMID: 26072116 Review.
-
FLIPPER, a combinatorial probe for correlated live imaging and electron microscopy, allows identification and quantitative analysis of various cells and organelles.Cell Tissue Res. 2015 Apr;360(1):61-70. doi: 10.1007/s00441-015-2142-7. Epub 2015 Mar 19. Cell Tissue Res. 2015. PMID: 25786736 Free PMC article.
-
In Vivo Time-Lapse Imaging and Analysis of Dendritic Structural Plasticity in Xenopus laevis Tadpoles.Cold Spring Harb Protoc. 2022 Jan 4;2022(1):pdb.prot106781. doi: 10.1101/pdb.prot106781. Cold Spring Harb Protoc. 2022. PMID: 33790043 Free PMC article.
-
Synaptic circuitry identified by intracellular labeling with horseradish peroxidase.J Electron Microsc Tech. 1990 Aug;15(4):369-76. doi: 10.1002/jemt.1060150406. J Electron Microsc Tech. 1990. PMID: 2202794 Review.
Cited by
-
Picking faces out of a crowd: genetic labels for identification of proteins in correlated light and electron microscopy imaging.Methods Cell Biol. 2012;111:139-55. doi: 10.1016/B978-0-12-416026-2.00008-X. Methods Cell Biol. 2012. PMID: 22857927 Free PMC article. Review.
-
Directed evolution of APEX2 for electron microscopy and proximity labeling.Nat Methods. 2015 Jan;12(1):51-4. doi: 10.1038/nmeth.3179. Epub 2014 Nov 24. Nat Methods. 2015. PMID: 25419960 Free PMC article.
-
Electron microscopy using the genetically encoded APEX2 tag in cultured mammalian cells.Nat Protoc. 2017 Sep;12(9):1792-1816. doi: 10.1038/nprot.2017.065. Epub 2017 Aug 10. Nat Protoc. 2017. PMID: 28796234 Free PMC article.
-
Reconstruction of genetically identified neurons imaged by serial-section electron microscopy.Elife. 2016 Jul 7;5:e15015. doi: 10.7554/eLife.15015. Elife. 2016. PMID: 27383271 Free PMC article.
-
In Vivo Analysis of the Neurovascular Niche in the Developing Xenopus Brain.eNeuro. 2017 Jul 31;4(4):ENEURO.0030-17.2017. doi: 10.1523/ENEURO.0030-17.2017. eCollection 2017 Jul-Aug. eNeuro. 2017. PMID: 28795134 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

