A human recombinant autoantibody-based immunotoxin specific for the fetal acetylcholine receptor inhibits rhabdomyosarcoma growth in vitro and in a murine transplantation model
- PMID: 20204062
- PMCID: PMC2829619
- DOI: 10.1155/2010/187621
A human recombinant autoantibody-based immunotoxin specific for the fetal acetylcholine receptor inhibits rhabdomyosarcoma growth in vitro and in a murine transplantation model
Abstract
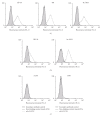
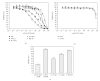
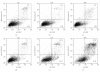
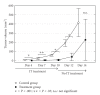
Rhabdomyosarcoma (RMS) is the most common malignant soft tissue tumor in children and is highly resistant to all forms of treatment currently available once metastasis or relapse has commenced. As it has recently been determined that the acetylcholine receptor (AChR) gamma-subunit, which defines the fetal AChR (fAChR) isoform, is almost exclusively expressed in RMS post partum, we recombinantly fused a single chain variable fragment (scFv) derived from a fully human anti-fAChR Fab-fragment to Pseudomonas exotoxin A to generate an anti-fAChR immunotoxin (scFv35-ETA). While scFv35-ETA had no damaging effect on fAChR-negative control cell lines, it killed human embryonic and alveolar RMS cell lines in vitro and delayed RMS development in a murine transplantation model. These results indicate that scFv35-ETA may be a valuable new therapeutic tool as well as a relevant step towards the development of a fully human immunotoxin directed against RMS. Moreover, as approximately 20% of metastatic malignant melanomas (MMs) display rhabdoid features including the expression of fAChR, the immunotoxin we developed may also prove to be of significant use in the treatment of these more common and most often fatal neoplasms.
Figures

Similar articles
-
Novel PSCA targeting scFv-fusion proteins for diagnosis and immunotherapy of prostate cancer.J Cancer Res Clin Oncol. 2017 Oct;143(10):2025-2038. doi: 10.1007/s00432-017-2472-9. Epub 2017 Jun 30. J Cancer Res Clin Oncol. 2017. PMID: 28667390
-
In vivo efficacy of the recombinant anti-CD64 immunotoxin H22(scFv)-ETA' in a human acute myeloid leukemia xenograft tumor model.Int J Cancer. 2011 Sep 1;129(5):1277-82. doi: 10.1002/ijc.25766. Epub 2011 Feb 26. Int J Cancer. 2011. PMID: 21077160
-
[Rhabdomyosarcoma lysis by T cells expressing a human autoantibody based chimeric receptor targeting the fetal acetylcholine receptors].Verh Dtsch Ges Pathol. 2006;90:264-76. Verh Dtsch Ges Pathol. 2006. PMID: 17867605 German.
-
Recombinant immunotoxins for the treatment of Hodgkin's disease (Review).Int J Mol Med. 2000 Nov;6(5):509-14. doi: 10.3892/ijmm.6.5.509. Int J Mol Med. 2000. PMID: 11029515 Review.
-
Quantification of immunotoxin number for complete therapeutic response.Methods Mol Biol. 2001;166:111-23. doi: 10.1385/1-59259-114-0:111. Methods Mol Biol. 2001. PMID: 11217362 Review. No abstract available.
Cited by
-
scFv antibody: principles and clinical application.Clin Dev Immunol. 2012;2012:980250. doi: 10.1155/2012/980250. Epub 2012 Mar 15. Clin Dev Immunol. 2012. PMID: 22474489 Free PMC article. Review.
-
Survivin blockade sensitizes rhabdomyosarcoma cells for lysis by fetal acetylcholine receptor-redirected T cells.Am J Pathol. 2013 Jun;182(6):2121-31. doi: 10.1016/j.ajpath.2013.02.017. Epub 2013 Apr 2. Am J Pathol. 2013. PMID: 23562272 Free PMC article.
-
Single-Chain Fragment Variable: Recent Progress in Cancer Diagnosis and Therapy.Cancers (Basel). 2022 Aug 30;14(17):4206. doi: 10.3390/cancers14174206. Cancers (Basel). 2022. PMID: 36077739 Free PMC article. Review.
-
Construction and preliminary identification of a prokaryotic expression single-chain antibody fragments library against Streptococcus pneumoniae from antibody-producing cells in human tonsil.Bioengineered. 2019 Dec;10(1):162-171. doi: 10.1080/21655979.2019.1616492. Bioengineered. 2019. PMID: 31088324 Free PMC article.
-
In vitro effects and ex vivo binding of an EGFR-specific immunotoxin on rhabdomyosarcoma cells.J Cancer Res Clin Oncol. 2015 Jun;141(6):1049-61. doi: 10.1007/s00432-014-1884-z. Epub 2014 Nov 30. J Cancer Res Clin Oncol. 2015. PMID: 25433506
References
-
- Parham DM, Ellison DA. Rhabdomyosarcomas in adults and children: an update. Archives of Pathology and Laboratory Medicine. 2006;130(10):1454–1465. - PubMed
-
- Koscielniak E, Morgan M, Treuner J. Soft tissue sarcoma in children: prognosis and management. Pediatric Drugs. 2002;4(1):21–28. - PubMed
-
- Raney RB, Anderson JR, Barr FG, et al. Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of intergroup rhabdomyosarcoma study group experience and rationale for intergroup rhabdomyosarcoma study V. Journal of Pediatric Hematology/Oncology. 2001;23(4):215–220. - PubMed
-
- Smith LM, Anderson JR, Coffin CM. Cytodifferentiation and clinical outcome after chemotherapy and radiation therapy for rhabdomyosarcoma (RMS) Medical and Pediatric Oncology. 2002;38:398–404. - PubMed
-
- Carli M, Colombatti R, Oberlin O, et al. European intergroup studies (MMT4-89 and MMT4-91) on childhood metastatic rhabdomyosarcoma: final results and analysis of prognostic factors. Journal of Clinical Oncology. 2004;22(23):4735–4742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

