Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability
- PMID: 20203192
- PMCID: PMC6634099
- DOI: 10.1523/JNEUROSCI.5098-09.2010
Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability
Abstract
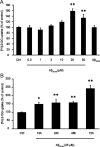
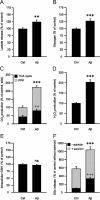
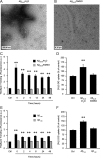
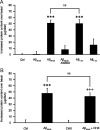
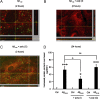
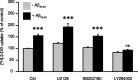
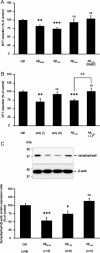
Amyloid-beta (Abeta) peptides play a key role in the pathogenesis of Alzheimer's disease and exert various toxic effects on neurons; however, relatively little is known about their influence on glial cells. Astrocytes play a pivotal role in brain homeostasis, contributing to the regulation of local energy metabolism and oxidative stress defense, two aspects of importance for neuronal viability and function. In the present study, we explored the effects of Abeta peptides on glucose metabolism in cultured astrocytes. Following Abeta(25-35) exposure, we observed an increase in glucose uptake and its various metabolic fates, i.e., glycolysis (coupled to lactate release), tricarboxylic acid cycle, pentose phosphate pathway, and incorporation into glycogen. Abeta increased hydrogen peroxide production as well as glutathione release into the extracellular space without affecting intracellular glutathione content. A causal link between the effects of Abeta on glucose metabolism and its aggregation and internalization into astrocytes through binding to members of the class A scavenger receptor family could be demonstrated. Using astrocyte-neuron cocultures, we observed that the overall modifications of astrocyte metabolism induced by Abeta impair neuronal viability. The effects of the Abeta(25-35) fragment were reproduced by Abeta(1-42) but not by Abeta(1-40). Finally, the phosphoinositide 3-kinase (PI3-kinase) pathway appears to be crucial in these events since both the changes in glucose utilization and the decrease in neuronal viability are prevented by LY294002, a PI3-kinase inhibitor. This set of observations indicates that Abeta aggregation and internalization into astrocytes profoundly alter their metabolic phenotype with deleterious consequences for neuronal viability.
Figures
Similar articles
-
The participation of insulin-like growth factor-binding protein 3 released by astrocytes in the pathology of Alzheimer's disease.Mol Brain. 2015 Dec 4;8(1):82. doi: 10.1186/s13041-015-0174-2. Mol Brain. 2015. PMID: 26637371 Free PMC article.
-
Effects of ketone bodies in Alzheimer's disease in relation to neural hypometabolism, β-amyloid toxicity, and astrocyte function.J Neurochem. 2015 Jul;134(1):7-20. doi: 10.1111/jnc.13107. Epub 2015 Apr 23. J Neurochem. 2015. PMID: 25832906 Review.
-
Nrf2 activation through the PI3K/GSK-3 axis protects neuronal cells from Aβ-mediated oxidative and metabolic damage.Alzheimers Res Ther. 2020 Jan 13;12(1):13. doi: 10.1186/s13195-019-0578-9. Alzheimers Res Ther. 2020. PMID: 31931869 Free PMC article.
-
Rescue of Abeta(1-42)-induced memory impairment in day-old chick by facilitation of astrocytic oxidative metabolism: implications for Alzheimer's disease.J Neurochem. 2009 May;109 Suppl 1:230-6. doi: 10.1111/j.1471-4159.2009.05800.x. J Neurochem. 2009. PMID: 19393032
-
A unifying hypothesis of Alzheimer's disease. IV. Causation and sequence of events.Rev Neurosci. 2000;11 Spec No:213-328. doi: 10.1515/revneuro.2000.11.s1.213. Rev Neurosci. 2000. PMID: 11065271 Review.
Cited by
-
Role of scavenger receptors in glia-mediated neuroinflammatory response associated with Alzheimer's disease.Mediators Inflamm. 2013;2013:895651. doi: 10.1155/2013/895651. Epub 2013 May 7. Mediators Inflamm. 2013. PMID: 23737655 Free PMC article. Review.
-
GFAP isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer disease.PLoS One. 2012;7(8):e42823. doi: 10.1371/journal.pone.0042823. Epub 2012 Aug 13. PLoS One. 2012. PMID: 22912745 Free PMC article.
-
Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes.J Clin Invest. 2012 Nov;122(11):3900-13. doi: 10.1172/JCI64102. Epub 2012 Oct 15. J Clin Invest. 2012. PMID: 23064363 Free PMC article.
-
Redistribution of metabolic resources through astrocyte networks mitigates neurodegenerative stress.Proc Natl Acad Sci U S A. 2020 Aug 4;117(31):18810-18821. doi: 10.1073/pnas.2009425117. Epub 2020 Jul 20. Proc Natl Acad Sci U S A. 2020. PMID: 32690710 Free PMC article.
-
Reactive astrocytes: The nexus of pathological and clinical hallmarks of Alzheimer's disease.Ageing Res Rev. 2021 Jul;68:101335. doi: 10.1016/j.arr.2021.101335. Epub 2021 Mar 31. Ageing Res Rev. 2021. PMID: 33812051 Free PMC article. Review.
References
-
- Akiyama H, Mori H, Saido T, Kondo H, Ikeda K, McGeer PL. Occurrence of the diffuse amyloid beta-protein (Abeta) deposits with numerous Abeta-containing glial cells in the cerebral cortex of patients with Alzheimer's disease. Glia. 1999;25:324–331. - PubMed
-
- Alarcon R, Fuenzalida C, Santibanez M, von Bernhardi R. Expression of scavenger receptors in glial cells: comparing the adhesion of astrocytes and microglia from neonatal rats to surface-bound beta-amyloid. J Biol Chem. 2005;280:30406–30415. - PubMed
-
- Allaman I, Pellerin L, Magistretti PJ. Glucocorticoids modulate neurotransmitter-induced glycogen metabolism in cultured cortical astrocytes. J Neurochem. 2004;88:900–908. - PubMed
-
- Bahia PK, Rattray M, Williams RJ. Dietary flavonoid (-)epicatechin stimulates phosphatidylinositol 3-kinase-dependent anti-oxidant response element activity and up-regulates glutathione in cortical astrocytes. J Neurochem. 2008;106:2194–2204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
