The Puf-family RNA-binding protein PfPuf2 regulates sexual development and sex differentiation in the malaria parasite Plasmodium falciparum
- PMID: 20197405
- PMCID: PMC2844316
- DOI: 10.1242/jcs.059824
The Puf-family RNA-binding protein PfPuf2 regulates sexual development and sex differentiation in the malaria parasite Plasmodium falciparum
Abstract
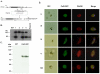
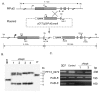
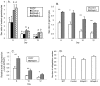
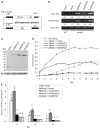
Translation regulation plays an important role during gametocytogenesis in the malaria parasite, a process that is obligatory for the transmission of the parasite through mosquito vectors. In this study we determined the function of PfPuf2, a member of the Puf family of translational repressors, in gametocytogenesis of Plasmodium falciparum. Tagging of the endogenous PfPuf2 protein with green fluorescent protein showed that PfPuf2 was expressed in both male and female gametocytes, and the protein was localized in the cytoplasm of the parasite. Targeted disruption of the PfPuf2 gene did not affect asexual growth of the parasite, but promoted the formation of gametocytes and differentiation of male gametocytes. Complementation studies were performed to confirm that the resultant phenotypic changes were due to disruption of the PfPuf2 gene. Episomal expression of PfPuf2 under its cognate promoter almost restored the gametocytogenesis rate in a PfPuf2 disruptant to the level of the wild-type parasite. It also partially restored the effect of PfPuf2 disruption on male-female sex ratio. In addition, episomal overexpression of PfPuf2 under its cognate promoter but with a higher concentration of the selection drug or under the constitutive hsp86 promoter in both the PfPuf2-disruptant and wild-type 3D7 lines, further dramatically reduced gametocytogenesis rates and sex ratios. These findings suggest that in this early branch of eukaryotes the function of PfPuf2 is consistent with the ancestral function of suppressing differentiation proposed for Puf-family proteins.
Figures

Similar articles
-
The malaria parasite Plasmodium falciparum encodes members of the Puf RNA-binding protein family with conserved RNA binding activity.Nucleic Acids Res. 2002 Nov 1;30(21):4607-17. doi: 10.1093/nar/gkf600. Nucleic Acids Res. 2002. PMID: 12409450 Free PMC article.
-
The RNA-binding protein Puf1 functions in the maintenance of gametocytes in Plasmodium falciparum.J Cell Sci. 2016 Aug 15;129(16):3144-52. doi: 10.1242/jcs.186908. Epub 2016 Jul 6. J Cell Sci. 2016. PMID: 27383769 Free PMC article.
-
Characterization of PfPuf2, member of the Puf family RNA-binding proteins from the malaria parasite Plasmodium falciparum.DNA Cell Biol. 2004 Nov;23(11):753-60. doi: 10.1089/dna.2004.23.753. DNA Cell Biol. 2004. PMID: 15585133
-
Plasmodium falciparum gametocytes: still many secrets of a hidden life.Mol Microbiol. 2007 Oct;66(2):291-302. doi: 10.1111/j.1365-2958.2007.05904.x. Epub 2007 Sep 3. Mol Microbiol. 2007. PMID: 17784927 Review.
-
Pleiotropic roles of cold shock proteins with special emphasis on unexplored cold shock protein member of Plasmodium falciparum.Malar J. 2020 Oct 27;19(1):382. doi: 10.1186/s12936-020-03448-6. Malar J. 2020. PMID: 33109193 Free PMC article. Review.
Cited by
-
Gametocyte Sex Ratio: The Key to Understanding Plasmodium falciparum Transmission?Trends Parasitol. 2019 Mar;35(3):226-238. doi: 10.1016/j.pt.2018.12.001. Epub 2018 Dec 26. Trends Parasitol. 2019. PMID: 30594415 Free PMC article. Review.
-
Alternative Splicing in Apicomplexan Parasites.mBio. 2019 Feb 19;10(1):e02866-18. doi: 10.1128/mBio.02866-18. mBio. 2019. PMID: 30782661 Free PMC article. Review.
-
Specific interaction of an RNA-binding protein with the 3'-UTR of its target mRNA is critical to oomycete sexual reproduction.PLoS Pathog. 2021 Oct 14;17(10):e1010001. doi: 10.1371/journal.ppat.1010001. eCollection 2021 Oct. PLoS Pathog. 2021. PMID: 34648596 Free PMC article.
-
A unique GCN5 histone acetyltransferase complex controls erythrocyte invasion and virulence in the malaria parasite Plasmodium falciparum.PLoS Pathog. 2021 Aug 17;17(8):e1009351. doi: 10.1371/journal.ppat.1009351. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34403450 Free PMC article.
-
Transition of Plasmodium sporozoites into liver stage-like forms is regulated by the RNA binding protein Pumilio.PLoS Pathog. 2011 May;7(5):e1002046. doi: 10.1371/journal.ppat.1002046. Epub 2011 May 19. PLoS Pathog. 2011. PMID: 21625527 Free PMC article.
References
-
- Alano P. (2007). Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol. Microbiol. 66, 291-302 - PubMed
-
- Alano P., Premawansa S., Bruce M. C., Carter R. (1991). A stage specific gene expressed at the onset of gametocytogenesis in Plasmodium falciparum. Mol. Biochem. Parasitol. 46, 81-88 - PubMed
-
- Asaoka-Taguchi M., Yamada M., Nakamura A., Hanyu K., Kobayashi S. (1999). Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nat. Cell Biol. 1, 431-437 - PubMed
-
- Bhattacharyya M. K., Kumar N. (2001). Effect of xanthurenic acid on infectivity of Plasmodium falciparum to Anopheles stephensi. Int. J. Parasitol. 31, 1129-1133 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

