Targeting and processing of site-specific DNA interstrand crosslinks
- PMID: 20196133
- PMCID: PMC2895014
- DOI: 10.1002/em.20557
Targeting and processing of site-specific DNA interstrand crosslinks
Abstract
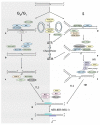
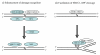
DNA interstrand crosslinks (ICLs) are among the most cytotoxic types of DNA damage, and thus ICL-inducing agents such as cyclophosphamide, melphalan, cisplatin, psoralen, and mitomycin C have been used clinically as anticancer drugs for decades. ICLs can also be formed endogenously as a consequence of cellular metabolic processes. ICL-inducing agents continue to be among the most effective chemotherapeutic treatments for many cancers; however, treatment with these agents can lead to secondary malignancies, in part due to mutagenic processing of the DNA lesions. The mechanisms of ICL repair have been characterized more thoroughly in bacteria and yeast than in mammalian cells. Thus, a better understanding of the molecular mechanisms of ICL processing offers the potential to improve the efficacy of these drugs in cancer therapy. In mammalian cells, it is thought that ICLs are repaired by the coordination of proteins from several pathways, including nucleotide excision repair (NER), base excision repair (BER), mismatch repair (MMR), homologous recombination (HR), translesion synthesis (TLS), and proteins involved in Fanconi anemia (FA). In this review, we focus on the potential functions of NER, MMR, and HR proteins in the repair of and response to ICLs in human cells and in mice. We will also discuss a unique approach, using psoralen covalently linked to triplex-forming oligonucleotides to direct ICLs to specific sites in the mammalian genome.
Environ. Mol. Mutagen. 2010. (c) 2010 Wiley-Liss, Inc.
Figures

Similar articles
-
Evidence for base excision repair processing of DNA interstrand crosslinks.Mutat Res. 2013 Mar-Apr;743-744:44-52. doi: 10.1016/j.mrfmmm.2012.11.007. Epub 2012 Dec 3. Mutat Res. 2013. PMID: 23219605 Free PMC article. Review.
-
Mismatch repair and nucleotide excision repair proteins cooperate in the recognition of DNA interstrand crosslinks.Nucleic Acids Res. 2009 Jul;37(13):4420-9. doi: 10.1093/nar/gkp399. Epub 2009 May 25. Nucleic Acids Res. 2009. PMID: 19468048 Free PMC article.
-
Multiple repair pathways mediate tolerance to chemotherapeutic cross-linking agents in vertebrate cells.Cancer Res. 2005 Dec 15;65(24):11704-11. doi: 10.1158/0008-5472.CAN-05-1214. Cancer Res. 2005. PMID: 16357182
-
Mismatch repair participates in error-free processing of DNA interstrand crosslinks in human cells.EMBO Rep. 2005 Jun;6(6):551-7. doi: 10.1038/sj.embor.7400418. EMBO Rep. 2005. PMID: 15891767 Free PMC article.
-
Translesion DNA synthesis polymerases in DNA interstrand crosslink repair.Environ Mol Mutagen. 2010 Jul;51(6):552-66. doi: 10.1002/em.20573. Environ Mol Mutagen. 2010. PMID: 20658647 Review.
Cited by
-
Targeted gene correction using psoralen, chlorambucil and camptothecin conjugates of triplex forming peptide nucleic acid (PNA).Artif DNA PNA XNA. 2011 Jan;2(1):23-32. doi: 10.4161/adna.2.1.15553. Artif DNA PNA XNA. 2011. PMID: 21686249 Free PMC article.
-
Targeting Chromosomal Architectural HMGB Proteins Could Be the Next Frontier in Cancer Therapy.Cancer Res. 2020 Jun 1;80(11):2075-2082. doi: 10.1158/0008-5472.CAN-19-3066. Epub 2020 Mar 9. Cancer Res. 2020. PMID: 32152151 Free PMC article. Review.
-
REV1 and DNA polymerase zeta in DNA interstrand crosslink repair.Environ Mol Mutagen. 2012 Dec;53(9):725-40. doi: 10.1002/em.21736. Epub 2012 Oct 13. Environ Mol Mutagen. 2012. PMID: 23065650 Free PMC article. Review.
-
Evidence for base excision repair processing of DNA interstrand crosslinks.Mutat Res. 2013 Mar-Apr;743-744:44-52. doi: 10.1016/j.mrfmmm.2012.11.007. Epub 2012 Dec 3. Mutat Res. 2013. PMID: 23219605 Free PMC article. Review.
-
Both hMutSα and hMutSß DNA mismatch repair complexes participate in 5-fluorouracil cytotoxicity.PLoS One. 2011;6(12):e28117. doi: 10.1371/journal.pone.0028117. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164234 Free PMC article.
References
-
- Aboussekhra A, Biggerstaff M, Shivji MK, Vilpo JA, Moncollin V, Podust VN, Protic M, Hubscher U, Egly JM, Wood RD. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell. 1995;80(6):859–68. - PubMed
-
- Aebi S, Fink D, Gordon R, Kim HK, Zheng H, Fink JL, Howell SB. Resistance to cytotoxic drugs in DNA mismatch repair-deficient cells. Clin Cancer Res. 1997;3(10):1763–7. - PubMed
-
- Aquilina G, Crescenzi M, Bignami M. Mismatch repair, G(2)/M cell cycle arrest and lethality after DNA damage. Carcinogenesis. 1999;20(12):2317–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

