Serine proteases and protease-activated receptor 2-dependent allodynia: a novel cancer pain pathway
- PMID: 20189717
- PMCID: PMC2861734
- DOI: 10.1016/j.pain.2010.02.010
Serine proteases and protease-activated receptor 2-dependent allodynia: a novel cancer pain pathway
Abstract
Mediators involved in the generation of pain in patients with cancer are poorly understood. Using a combined molecular, pharmacologic, behavioral, and genetic approach, we have identified a novel mechanism of cancer-dependent allodynia induced by protease-activated receptor 2 (PAR2). Here we show that human head and neck carcinoma cells have increased levels of proteolytic activity compared to normal human cell controls. Supernatant from human carcinoma cells, but not controls, caused marked and prolonged mechanical allodynia in mice, when administered into the hindpaw. This nociceptive effect was abolished by serine protease inhibition, diminished by mast cell depletion and absent in PAR2-deficient mice. In addition, non-contact co-culture of trigeminal ganglion neurons with human head and neck carcinoma cells increased the proportion of neurons that exhibited PAR2-immunoreactivity. Our results point to a direct role for serine proteases and their receptor in the pathogenesis of cancer pain. This previously unrecognized cancer pain pathway has important therapeutic implications wherein serine protease inhibitors and PAR2 antagonists may be useful for the treatment of cancer pain.
Copyright 2010 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest for this study.
Figures
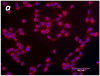








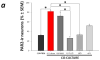


Similar articles
-
TMPRSS2, a novel membrane-anchored mediator in cancer pain.Pain. 2015 May;156(5):923-930. doi: 10.1097/j.pain.0000000000000130. Pain. 2015. PMID: 25734995 Free PMC article.
-
Serine proteases and protease-activated receptor 2 mediate the proinflammatory and algesic actions of diverse stimulants.Br J Pharmacol. 2014 Aug;171(16):3814-26. doi: 10.1111/bph.12738. Br J Pharmacol. 2014. PMID: 24749982 Free PMC article.
-
Involvement of mast cells and proteinase-activated receptor 2 in oxaliplatin-induced mechanical allodynia in mice.Pharmacol Res. 2016 Mar;105:84-92. doi: 10.1016/j.phrs.2016.01.008. Epub 2016 Jan 21. Pharmacol Res. 2016. PMID: 26804251
-
Protease-activated receptor 2 signalling pathways: a role in pain processing.Expert Opin Ther Targets. 2014 Jan;18(1):15-27. doi: 10.1517/14728222.2014.844792. Epub 2013 Oct 23. Expert Opin Ther Targets. 2014. PMID: 24147628 Review.
-
Membrane-Anchored Serine Proteases and Protease-Activated Receptor-2-Mediated Signaling: Co-Conspirators in Cancer Progression.Cancer Res. 2019 Jan 15;79(2):301-310. doi: 10.1158/0008-5472.CAN-18-1745. Epub 2019 Jan 4. Cancer Res. 2019. PMID: 30610085 Free PMC article. Review.
Cited by
-
Mechanisms of cancer pain.Front Pain Res (Lausanne). 2023 Jan 4;3:1030899. doi: 10.3389/fpain.2022.1030899. eCollection 2022. Front Pain Res (Lausanne). 2023. PMID: 36688083 Free PMC article. Review.
-
Pain: Persistent postsurgery and bone cancer-related pain.J Int Med Res. 2019 Feb;47(2):528-543. doi: 10.1177/0300060518818296. Epub 2019 Jan 11. J Int Med Res. 2019. PMID: 30632434 Free PMC article. Review.
-
Nanotechnology-based drug delivery systems for treatment of oral cancer: a review.Int J Nanomedicine. 2014 Aug 8;9:3719-35. doi: 10.2147/IJN.S61670. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25143724 Free PMC article. Review.
-
Protease-activated receptor 2 activation is sufficient to induce the transition to a chronic pain state.Pain. 2015 May;156(5):859-867. doi: 10.1097/j.pain.0000000000000125. Pain. 2015. PMID: 25734998 Free PMC article.
-
Recent advances in cancer pain management.F1000Prime Rep. 2014 Feb 3;6:10. doi: 10.12703/P6-10. eCollection 2014. F1000Prime Rep. 2014. PMID: 24592322 Free PMC article. Review.
References
-
- Aromando RF, Pérez MA, Heber EM, Trivillin VA, Tomasi VH, Schwint AE, Itoiz ME. Potential role of mast cells in hamster cheek pouch carcinogenesis. Oral Oncol. 2008;44:1080–1087. - PubMed
-
- Bjordal K, Ahlner-Elmqvist M, Hammerlid E, Boysen M, Evensen JF, Biörklund A, Jannert M, Westin T, Kaasa S. A prospective study of quality of life in head and neck cancer patients. Part II: Longitudinal data. Laryngoscope. 2001;111:1440–1452. - PubMed
-
- Brasseur L. Review of current pharmacologic treatment of pain. Drugs. 1997;53:10–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

