The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production
- PMID: 20181711
- PMCID: PMC2863746
- DOI: 10.1128/JVI.02487-09
The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production
Abstract
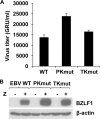
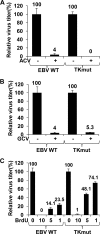
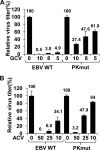
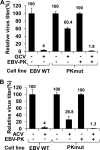
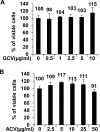
Ganciclovir (GCV) and acyclovir (ACV) are guanine nucleoside analogues that inhibit lytic herpesvirus replication. GCV and ACV must be monophosphorylated by virally encoded enzymes to be converted into nucleotides and incorporated into viral DNA. However, whether GCV and/or ACV phosphorylation in Epstein-Barr virus (EBV)-infected cells is mediated primarily by the EBV-encoded protein kinase (EBV-PK), the EBV-encoded thymidine kinase (EBV-TK), or both is controversial. To examine this question, we constructed EBV mutants containing stop codons in either the EBV-PK or EBV-TK open reading frame and selected for stable 293T clones latently infected with wild-type EBV or each of the mutant viruses. Cells were induced to the lytic form of viral replication with a BZLF1 expression vector in the presence and absence of various doses of GCV and ACV, and infectious viral titers were determined by a green Raji cell assay. As expected, virus production in wild-type EBV-infected 293T cells was inhibited by both GCV (50% inhibitory concentration [IC(50)] = 1.5 microM) and ACV (IC(50) = 4.1 microM). However, the EBV-PK mutant (which replicates as well as the wild-type (WT) virus in 293T cells) was resistant to both GCV (IC(50) = 19.6 microM) and ACV (IC(50) = 36.4 microM). Expression of the EBV-PK protein in trans restored GCV and ACV sensitivity in cells infected with the PK mutant virus. In contrast, in 293T cells infected with the TK mutant virus, viral replication remained sensitive to both GCV (IC(50) = 1.2 microM) and ACV (IC(50) = 2.8 microM), although susceptibility to the thymine nucleoside analogue, bromodeoxyuridine, was reduced. Thus, EBV-PK but not EBV-TK mediates ACV and GCV susceptibilities.
Figures

Similar articles
-
The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase.Antimicrob Agents Chemother. 1998 Nov;42(11):2923-31. doi: 10.1128/AAC.42.11.2923. Antimicrob Agents Chemother. 1998. PMID: 9797227 Free PMC article.
-
Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues.Antimicrob Agents Chemother. 2001 Jul;45(7):2082-91. doi: 10.1128/AAC.45.7.2082-2091.2001. Antimicrob Agents Chemother. 2001. PMID: 11408227 Free PMC article.
-
Analysis of phosphorylation pathways of antiherpesvirus nucleosides by varicella-zoster virus-specific enzymes.Antimicrob Agents Chemother. 1996 Apr;40(4):920-3. doi: 10.1128/AAC.40.4.920. Antimicrob Agents Chemother. 1996. PMID: 8849252 Free PMC article.
-
Perspectives on interactions of acyclovir with Epstein-Barr and other herpes viruses.Am J Med. 1982 Jul 20;73(1A):18-26. doi: 10.1016/0002-9343(82)90057-2. Am J Med. 1982. PMID: 6285710 Review.
-
[Studies on evaluation of natural products for antiviral effects and their applications].Yakugaku Zasshi. 2008 Jan;128(1):61-79. doi: 10.1248/yakushi.128.61. Yakugaku Zasshi. 2008. PMID: 18176057 Review. Japanese.
Cited by
-
Reactivation of Epstein-Barr Virus by HIF-1α Requires p53.J Virol. 2020 Aug 31;94(18):e00722-20. doi: 10.1128/JVI.00722-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641480 Free PMC article.
-
Lenalidomide, Thalidomide, and Pomalidomide Reactivate the Epstein-Barr Virus Lytic Cycle through Phosphoinositide 3-Kinase Signaling and Ikaros Expression.Clin Cancer Res. 2016 Oct 1;22(19):4901-4912. doi: 10.1158/1078-0432.CCR-15-2242. Epub 2016 Jun 13. Clin Cancer Res. 2016. PMID: 27297582 Free PMC article.
-
Key motifs in EBV (Epstein-Barr virus)-encoded protein kinase for phosphorylation activity and nuclear localization.Biochem J. 2010 Oct 15;431(2):227-35. doi: 10.1042/BJ20100558. Biochem J. 2010. PMID: 20704565 Free PMC article.
-
Feature Reviews of the Molecular Mechanisms of Nasopharyngeal Carcinoma.Biomedicines. 2023 May 25;11(6):1528. doi: 10.3390/biomedicines11061528. Biomedicines. 2023. PMID: 37371623 Free PMC article. Review.
-
Antineoplastic and anti-inflammatory effects of bortezomib on systemic chronic active EBV infection.Blood Adv. 2021 Apr 13;5(7):1805-1815. doi: 10.1182/bloodadvances.2020002417. Blood Adv. 2021. PMID: 33787860 Free PMC article.
References
-
- Ambinder, R. F. 2007. Epstein-Barr virus and Hodgkin lymphoma. Hematol. Am. Soc. Hematol. Educ. Prog. 2007:204-209. - PubMed
-
- Andersson, J., B. Skoldenberg, W. Henle, J. Giesecke, A. Ortqvist, I. Julander, E. Gustavsson, B. Akerlund, S. Britton, and I. Ernberg. 1987. Acyclovir treatment in infectious mononucleosis: a clinical and virological study. Infection 15(Suppl. 1):S14-S20. - PubMed
-
- Andersson, J. P. 1991. Clinical aspects on Epstein-Barr virus infection. Scand. J. Infect. Dis. Suppl. 80:94-104. - PubMed
-
- Balfour, H. H., Jr., K. M. Hokanson, R. M. Schacherer, C. M. Fietzer, D. O. Schmeling, C. J. Holman, H. E. Vezina, and R. C. Brundage. 2007. A virologic pilot study of valacyclovir in infectious mononucleosis. J. Clin. Virol. 39:16-21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

