A flow cytometry-based FRET assay to identify and analyse protein-protein interactions in living cells
- PMID: 20179761
- PMCID: PMC2825263
- DOI: 10.1371/journal.pone.0009344
A flow cytometry-based FRET assay to identify and analyse protein-protein interactions in living cells
Abstract
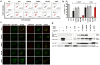
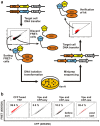
Background: Försters resonance energy transfer (FRET) microscopy is widely used for the analysis of protein interactions in intact cells. However, FRET microscopy is technically challenging and does not allow assessing interactions in large cell numbers. To overcome these limitations we developed a flow cytometry-based FRET assay and analysed interactions of human and simian immunodeficiency virus (HIV and SIV) Nef and Vpu proteins with cellular factors, as well as HIV Rev multimer-formation.
Results: Amongst others, we characterize the interaction of Vpu with CD317 (also termed Bst-2 or tetherin), a host restriction factor that inhibits HIV release from infected cells and demonstrate that the direct binding of both is mediated by the Vpu membrane-spanning region. Furthermore, we adapted our assay to allow the identification of novel protein interaction partners in a high-throughput format.
Conclusion: The presented combination of FRET and FACS offers the precious possibility to discover and define protein interactions in living cells and is expected to contribute to the identification of novel therapeutic targets for treatment of human diseases.
Conflict of interest statement
Figures



Similar articles
-
Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2.PLoS Pathog. 2009 May;5(5):e1000429. doi: 10.1371/journal.ppat.1000429. Epub 2009 May 15. PLoS Pathog. 2009. PMID: 19436700 Free PMC article.
-
Vpu of a Simian Immunodeficiency Virus Isolated from Greater Spot-Nosed Monkey Antagonizes Human BST-2 via Two AxxxxxxxW Motifs.J Virol. 2020 Jan 6;94(2):e01669-19. doi: 10.1128/JVI.01669-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31666374 Free PMC article.
-
Ancient adaptive evolution of tetherin shaped the functions of Vpu and Nef in human immunodeficiency virus and primate lentiviruses.J Virol. 2010 Jul;84(14):7124-34. doi: 10.1128/JVI.00468-10. Epub 2010 May 5. J Virol. 2010. PMID: 20444900 Free PMC article.
-
Antiviral activity of the interferon-induced cellular protein BST-2/tetherin.AIDS Res Hum Retroviruses. 2009 Dec;25(12):1197-210. doi: 10.1089/aid.2009.0253. AIDS Res Hum Retroviruses. 2009. PMID: 19929170 Free PMC article. Review.
-
Emerging role of the host restriction factor tetherin in viral immune sensing.J Mol Biol. 2013 Dec 13;425(24):4956-64. doi: 10.1016/j.jmb.2013.09.029. Epub 2013 Sep 26. J Mol Biol. 2013. PMID: 24075872 Review.
Cited by
-
HIV-1 Vpu affects the anterograde transport and the glycosylation pattern of NTB-A.Virology. 2013 Jun 5;440(2):190-203. doi: 10.1016/j.virol.2013.02.021. Epub 2013 Mar 22. Virology. 2013. PMID: 23528733 Free PMC article.
-
Evidence for a complex formation between CYP2J5 and mEH in living cells by FRET analysis of membrane protein interaction in the endoplasmic reticulum (FAMPIR).Arch Toxicol. 2017 Nov;91(11):3561-3570. doi: 10.1007/s00204-017-2072-0. Epub 2017 Oct 13. Arch Toxicol. 2017. PMID: 29030652 Free PMC article.
-
HIV-1 Nef and Vpu Interfere with L-Selectin (CD62L) Cell Surface Expression To Inhibit Adhesion and Signaling in Infected CD4+ T Lymphocytes.J Virol. 2015 May;89(10):5687-700. doi: 10.1128/JVI.00611-15. Epub 2015 Mar 11. J Virol. 2015. PMID: 25822027 Free PMC article.
-
Identification of novel key amino acids at the interface of the transmembrane domains of human BST-2 and HIV-1 Vpu.Retrovirology. 2013 Aug 6;10:84. doi: 10.1186/1742-4690-10-84. Retrovirology. 2013. PMID: 23919512 Free PMC article.
-
Inhibition of Tau seeding by targeting Tau nucleation core within neurons with a single domain antibody fragment.Mol Ther. 2022 Apr 6;30(4):1484-1499. doi: 10.1016/j.ymthe.2022.01.009. Epub 2022 Jan 7. Mol Ther. 2022. PMID: 35007758 Free PMC article.
References
-
- Piehler J. New methodologies for measuring protein interactions in vivo and in vitro. Curr Opin Struct Biol. 2005;15:4–14. - PubMed
-
- Selvin PR. The renaissance of fluorescence resonance energy transfer. Nat Struct Biol. 2000;7:730–734. - PubMed
-
- Piston DW, Kremers GJ. Fluorescent protein FRET: the good, the bad and the ugly. Trends Biochem Sci. 2007;32:407–414. - PubMed
-
- Yan Y, Marriott G. Analysis of protein interactions using fluorescence technologies. Curr Opin Chem Biol. 2003;7:635–640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

