DHHC5 interacts with PDZ domain 3 of post-synaptic density-95 (PSD-95) protein and plays a role in learning and memory
- PMID: 20178993
- PMCID: PMC2857114
- DOI: 10.1074/jbc.M109.079426
DHHC5 interacts with PDZ domain 3 of post-synaptic density-95 (PSD-95) protein and plays a role in learning and memory
Abstract
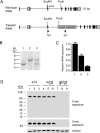
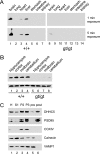
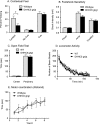
A family of integral membrane proteins containing a signature DHHC motif has been shown to display protein S-acyltransferase activity, modifying cysteine residues in proteins with fatty acids. The physiological roles of these proteins have largely been unexplored. Here we report that mice homozygous for a hypomorphic allele of a previously uncharacterized member, DHHC5, are born at half the expected rate, and survivors show a marked deficit in contextual fear conditioning, an indicator of defective hippocampal-dependent learning. DHHC5 is highly enriched in a post-synaptic density preparation and co-immunoprecipitates with post-synaptic density protein-95 (PSD-95), an interaction that is mediated through binding of the carboxyl terminus of DHHC5 and the PDZ3 domain of PSD-95. Immunohistochemistry demonstrated that DHHC5 is expressed in the CA3 and dentate gyrus in the hippocampus. These findings point to a previously unsuspected role for DHHC5 in post-synaptic function affecting learning and memory.
Figures


Similar articles
-
Impaired synaptic clustering of postsynaptic density proteins and altered signal transmission in hippocampal neurons, and disrupted learning behavior in PDZ1 and PDZ2 ligand binding-deficient PSD-95 knockin mice.Mol Brain. 2012 Dec 26;5:43. doi: 10.1186/1756-6606-5-43. Mol Brain. 2012. PMID: 23268962 Free PMC article.
-
Essential contribution of the ligand-binding beta B/beta C loop of PDZ1 and PDZ2 in the regulation of postsynaptic clustering, scaffolding, and localization of postsynaptic density-95.J Neurosci. 2006 Jan 18;26(3):763-74. doi: 10.1523/JNEUROSCI.2489-05.2006. J Neurosci. 2006. PMID: 16421296 Free PMC article.
-
Activity-regulated trafficking of the palmitoyl-acyl transferase DHHC5.Nat Commun. 2015 Sep 3;6:8200. doi: 10.1038/ncomms9200. Nat Commun. 2015. PMID: 26334723 Free PMC article.
-
PDZ Ligand Binding-Induced Conformational Coupling of the PDZ-SH3-GK Tandems in PSD-95 Family MAGUKs.J Mol Biol. 2018 Jan 5;430(1):69-86. doi: 10.1016/j.jmb.2017.11.003. Epub 2017 Nov 11. J Mol Biol. 2018. PMID: 29138001
-
Preso, a novel PSD-95-interacting FERM and PDZ domain protein that regulates dendritic spine morphogenesis.J Neurosci. 2008 Dec 31;28(53):14546-56. doi: 10.1523/JNEUROSCI.3112-08.2008. J Neurosci. 2008. PMID: 19118189 Free PMC article.
Cited by
-
S-acylated Golga7b stabilises DHHC5 at the plasma membrane to regulate cell adhesion.EMBO Rep. 2019 Oct 4;20(10):e47472. doi: 10.15252/embr.201847472. Epub 2019 Aug 12. EMBO Rep. 2019. PMID: 31402609 Free PMC article.
-
Massive endocytosis triggered by surface membrane palmitoylation under mitochondrial control in BHK fibroblasts.Elife. 2013 Nov 26;2:e01293. doi: 10.7554/eLife.01293. Elife. 2013. PMID: 24282236 Free PMC article.
-
Roles of palmitoylation in axon growth, degeneration and regeneration.J Neurosci Res. 2017 Aug;95(8):1528-1539. doi: 10.1002/jnr.24003. Epub 2017 Feb 2. J Neurosci Res. 2017. PMID: 28150429 Free PMC article. Review.
-
Crosstalk of Synapsin1 palmitoylation and phosphorylation controls the dynamicity of synaptic vesicles in neurons.Cell Death Dis. 2022 Sep 12;13(9):786. doi: 10.1038/s41419-022-05235-4. Cell Death Dis. 2022. PMID: 36097267 Free PMC article.
-
Palmitoylation of A-kinase anchoring protein 79/150 regulates dendritic endosomal targeting and synaptic plasticity mechanisms.J Neurosci. 2012 May 23;32(21):7119-36. doi: 10.1523/JNEUROSCI.0784-12.2012. J Neurosci. 2012. PMID: 22623657 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

