Immunotherapy markedly increases the effectiveness of antimicrobial therapy for treatment of Burkholderia pseudomallei infection
- PMID: 20176901
- PMCID: PMC2863600
- DOI: 10.1128/AAC.01513-09
Immunotherapy markedly increases the effectiveness of antimicrobial therapy for treatment of Burkholderia pseudomallei infection
Abstract
Burkholderia pseudomallei is a soil bacterium that is endemic in southeast Asia and northern Australia and that can cause both acutely lethal pneumonia and chronic systemic infections in humans. The effective treatment of infection with B. pseudomallei requires rapid diagnosis and prolonged treatment with high doses of antimicrobials, and even with appropriate antibiotic therapy, patient relapses are common. Thus, new approaches to the treatment of B. pseudomallei infections are needed. In the present study, we asked whether active immunotherapy with gamma interferon (IFN-gamma), a key cytokine regulating the intracellular replication of B. pseudomallei, could increase the effectiveness of conventional antimicrobial therapy for B. pseudomallei infection. Macrophage infection assays and in vivo pulmonary challenge models were used to assess the inhibitory effects of combined treatment with IFN-gamma and ceftazidime on B. pseudomallei infection. We found that treatment with even very low doses of IFN-gamma and ceftazidime elicited strong synergistic inhibition of B. pseudomallei growth within infected macrophages. In vivo, active immunotherapy markedly potentiated the effectiveness of low-dose ceftazidime therapy for the treatment of infected mice in a pulmonary challenge model of B. pseudomallei. Combined treatment was associated with a significant reduction in the bacterial burden and a significant lessening of bacterial dissemination. We concluded, therefore, that immunotherapy with either endogenous or exogenous IFN-gamma could significantly increase the effectiveness of conventional antimicrobial therapy for the treatment of acute B. pseudomallei infection.
Figures
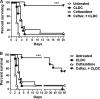
Comment in
-
Gamma interferon supplementation for melioidosis.Antimicrob Agents Chemother. 2010 Oct;54(10):4520; author reply 4520-1. doi: 10.1128/AAC.00805-10. Antimicrob Agents Chemother. 2010. PMID: 20852188 Free PMC article. No abstract available.
Similar articles
-
Gamma interferon supplementation for melioidosis.Antimicrob Agents Chemother. 2010 Oct;54(10):4520; author reply 4520-1. doi: 10.1128/AAC.00805-10. Antimicrob Agents Chemother. 2010. PMID: 20852188 Free PMC article. No abstract available.
-
Activity of tigecycline in the treatment of acute Burkholderia pseudomallei infection in a murine model.Int J Antimicrob Agents. 2006 Nov;28(5):460-4. doi: 10.1016/j.ijantimicag.2006.07.022. Int J Antimicrob Agents. 2006. PMID: 17046208
-
Combination antimicrobial therapy of acute Burkholderia pseudomallei infection in a mouse model.Pathology. 1999 Aug;31(3):264-7. doi: 10.1080/003130299105106. Pathology. 1999. PMID: 10503275
-
Melioidosis.Nat Rev Dis Primers. 2018 Feb 1;4:17107. doi: 10.1038/nrdp.2017.107. Nat Rev Dis Primers. 2018. PMID: 29388572 Free PMC article. Review.
-
Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis.Future Microbiol. 2012 Dec;7(12):1389-99. doi: 10.2217/fmb.12.116. Future Microbiol. 2012. PMID: 23231488 Free PMC article. Review.
Cited by
-
Dysregulated immunologic landscape of the early host response in melioidosis.JCI Insight. 2024 Aug 20;9(18):e179106. doi: 10.1172/jci.insight.179106. JCI Insight. 2024. PMID: 39163129 Free PMC article.
-
Ceftazidime is a potential drug to inhibit cell proliferation by increasing cellular p27.J Antibiot (Tokyo). 2024 Oct;77(10):697-705. doi: 10.1038/s41429-024-00751-1. Epub 2024 Jun 19. J Antibiot (Tokyo). 2024. PMID: 38898184
-
Evaluation of Immune Nanoparticles for Rapid and Non-Specific Activation of Antiviral and Antibacterial Immune Responses in Cattle, Swine, and Poultry.Animals (Basel). 2023 May 18;13(10):1686. doi: 10.3390/ani13101686. Animals (Basel). 2023. PMID: 37238119 Free PMC article.
-
Efficacy of ceftazidime in a murine model following a lethal aerosol exposure to Burkholderia pseudomallei.Sci Rep. 2023 Mar 10;13(1):4047. doi: 10.1038/s41598-023-31131-8. Sci Rep. 2023. PMID: 36899021 Free PMC article.
-
Dietary antioxidant seleno-L-methionine protects macrophages infected with Burkholderia thailandensis.PLoS One. 2020 Sep 3;15(9):e0238174. doi: 10.1371/journal.pone.0238174. eCollection 2020. PLoS One. 2020. PMID: 32881891 Free PMC article.
References
-
- Arjcharoen, S., C. Wikraiphat, M. Pudla, K. Limposuwan, D. E. Woods, S. Sirisinha, and P. Utaisincharoen. 2007. Fate of a Burkholderia pseudomallei lipopolysaccharide mutant in the mouse macrophage cell line RAW 264.7: possible role for the O-antigenic polysaccharide moiety of lipopolysaccharide in internalization and intracellular survival. Infect. Immun. 75:4298-4304. - PMC - PubMed
-
- Cheng, A. C., P. Dasari, and B. J. Currie. 2004. Granulocyte colony-stimulating factor and an in vitro whole blood model of melioidosis. Eur. J. Clin. Microbiol. Infect. Dis. 23:205-207. - PubMed
-
- Cheng, A. C., D. P. Stephens, N. M. Anstey, and B. J. Currie. 2004. Adjunctive granulocyte colony-stimulating factor for treatment of septic shock due to melioidosis. Clin. Infect. Dis. 38:32-37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

