Point of care strategy for rapid diagnosis of novel A/H1N1 influenza virus
- PMID: 20174646
- PMCID: PMC2822848
- DOI: 10.1371/journal.pone.0009215
Point of care strategy for rapid diagnosis of novel A/H1N1 influenza virus
Erratum in
- PLoS One. 2010;5(4). doi: 10.1371/annotation/5c0b32ed-3c18-4634-9f59-f5968e91710f
Abstract
Background: Within months of the emergence of the novel A/H1N1 pandemic influenza virus (nA/H1N1v), systematic screening for the surveillance of the pandemic was abandoned in France and in some other countries. At the end of June 2009, we implemented, for the public hospitals of Marseille, a Point Of Care (POC) strategy for rapid diagnosis of the novel A/H1N1 influenza virus, in order to maintain local surveillance and to evaluate locally the kinetics of the pandemic.
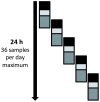
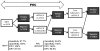
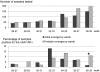
Methodology/principal findings: Two POC laboratories, located in strategic places, were organized to receive and test samples 24 h/24. POC strategy consisted of receiving and processing naso-pharyngeal specimens in preparation for the rapid influenza diagnostic test (RIDT) and real-time RT-PCR assay (rtRT-PCR). This strategy had the theoretical capacity of processing up to 36 samples per 24 h. When the flow of samples was too high, the rtRT-PCR test was abandoned in the POC laboratories and transferred to the core virology laboratory. Confirmatory diagnosis was performed in the core virology laboratory twice a day using two distinct rtRT-PCR techniques that detect either influenza A virus or nA/N1N1v. Over a period of three months, 1974 samples were received in the POC laboratories, of which 111 were positive for nA/H1N1v. Specificity and sensitivity of RIDT were 100%, and 57.7% respectively. Positive results obtained using RIDT were transmitted to clinical practitioners in less than 2 hours. POC processed rtRT-PCR results were available within 7 hours, and rtRT-PCR confirmation within 24 hours.
Conclusions/significance: The POC strategy is of benefit, in all cases (with or without rtRT-PCR assay), because it provides continuous reception/processing of samples and reduction of the time to provide consolidated results to the clinical practitioners. We believe that implementation of the POC strategy for the largest number of suspect cases may improve the quality of patient care and our knowledge of the epidemiology of the pandemic.
Conflict of interest statement
Figures


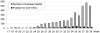
Update of
-
Point of care strategy for rapid diagnosis of novel A/H1N1 influenza virus.PLoS Curr. 2009 Sep 21;1:RRN1039. doi: 10.1371/currents.rrn1039. PLoS Curr. 2009. Update in: PLoS One. 2010 Feb 17;5(2):e9215. doi: 10.1371/journal.pone.0009215 PMID: 20025202 Free PMC article. Updated.
Similar articles
-
Comparison of the performance of direct fluorescent antibody staining, a point-of-care rapid antigen test and virus isolation with that of RT-PCR for the detection of novel 2009 influenza A (H1N1) virus in respiratory specimens.J Med Microbiol. 2010 Jun;59(Pt 6):713-717. doi: 10.1099/jmm.0.017244-0. Epub 2010 Mar 4. J Med Microbiol. 2010. PMID: 20203216
-
Development of a real-time RT-PCR for the detection of swine-lineage influenza A (H1N1) virus infections.J Clin Virol. 2009 Jul;45(3):196-9. doi: 10.1016/j.jcv.2009.06.001. Epub 2009 Jun 10. J Clin Virol. 2009. PMID: 19540799 Free PMC article.
-
Novel virus influenza A (H1N1sw) in South-Eastern France, April-August 2009.PLoS One. 2010 Feb 17;5(2):e9214. doi: 10.1371/journal.pone.0009214. PLoS One. 2010. PMID: 20174643 Free PMC article.
-
Tools to detect influenza virus.Yonsei Med J. 2013 May 1;54(3):560-6. doi: 10.3349/ymj.2013.54.3.560. Yonsei Med J. 2013. PMID: 23549796 Free PMC article. Review.
-
[Pandemic influenza A/H1N1 2009 : Challenge for intensive care medicine].Anaesthesist. 2010 Jan;59(1):11-22. doi: 10.1007/s00101-009-1667-0. Anaesthesist. 2010. PMID: 20107944 Free PMC article. Review. German.
Cited by
-
Revolutionizing clinical microbiology laboratory organization in hospitals with in situ point-of-care.PLoS One. 2011;6(7):e22403. doi: 10.1371/journal.pone.0022403. Epub 2011 Jul 19. PLoS One. 2011. PMID: 21811599 Free PMC article.
-
Development of a Specific and Rapid Diagnostic Method for Detecting Influenza A (H1N1) pdm09 Virus Infection Using Immunochromatographic Assay.Osong Public Health Res Perspect. 2013 Dec;4(6):342-6. doi: 10.1016/j.phrp.2013.10.006. Epub 2013 Nov 7. Osong Public Health Res Perspect. 2013. PMID: 24524023 Free PMC article.
-
A meta-analysis of point-of-care laboratory tests in the diagnosis of novel 2009 swine-lineage pandemic influenza A (H1N1).Diagn Microbiol Infect Dis. 2011 Apr;69(4):410-8. doi: 10.1016/j.diagmicrobio.2010.10.009. Diagn Microbiol Infect Dis. 2011. PMID: 21396538 Free PMC article.
-
[Interests and limitations of rapid diagnostic tests for respiratory and gastrointestinal viral diseases].Rev Francoph Lab. 2015 Jul-Aug;2015(474):45-50. doi: 10.1016/S1773-035X(15)30200-8. Epub 2015 Jul 10. Rev Francoph Lab. 2015. PMID: 32288822 Free PMC article. French.
-
Infectious Disease Management through Point-of-Care Personalized Medicine Molecular Diagnostic Technologies.J Pers Med. 2012 May 2;2(2):50-70. doi: 10.3390/jpm2020050. J Pers Med. 2012. PMID: 25562799 Free PMC article. Review.
References
-
- World Health Organization. Statement to the press by WHO Director-General Dr Margaret Chan. 2009. Available: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6.... Accessed on 2009 Aug. 15.
-
- Levy-Bruhl D, Vaux S. Modified surveillance of influenza A(H1N1)v virus infections in France. Euro Surveill. 2009;14 - PubMed
-
- InVS: Institut de Veille Sanitaire, France. Bulletin épidémiologique grippe A (H1N1) 2009. Point au 29 septembre 2009 à 11 h. 2009. Available: http://www.invs.sante.fr/display/?doc=surveillance/grippe_dossier/index_.... Accessed on 2009 Oct. 20.
-
- Belgian working group on influenza A(H1N1)v. Influenza A(H1N1)v virus infections in Belgium, May-June 2009. Euro Surveill. 2009;14 - PubMed
-
- Lytras T, Theocharopoulos G, Tsiodras S, Mentis A, Panagiotopoulos T, et al. Enhanced surveillance of influenza A(H1N1)v in Greece during the containment phase. Euro Surveill. 2009;14 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

