Recombinant myostatin (GDF-8) propeptide enhances the repair and regeneration of both muscle and bone in a model of deep penetrant musculoskeletal injury
- PMID: 20173658
- PMCID: PMC3738012
- DOI: 10.1097/TA.0b013e3181c451f4
Recombinant myostatin (GDF-8) propeptide enhances the repair and regeneration of both muscle and bone in a model of deep penetrant musculoskeletal injury
Abstract
Background: Myostatin (GDF-8) is known as a potent inhibitor of muscle growth and development, and myostatin is also expressed early in the fracture healing process. The purpose of this study was to test the hypothesis that a new myostatin inhibitor, a recombinant myostatin propeptide, can enhance the repair and regeneration of both muscle and bone in cases of deep penetrant injury.
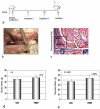
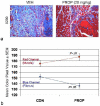
Methods: We used a fibula osteotomy model with associated damage to lateral compartment muscles (fibularis longus and brevis) in mice to test the hypothesis that blocking active myostatin with systemic injections of a recombinant myostatin propeptide would improve muscle and bone repair. Mice were assigned to two treatment groups after undergoing a fibula osteotomy: those receiving either vehicle (saline) or recombinant myostatin propeptide (20 mg/kg). Mice received one injection on the day of surgery, another injection 5 days after surgery, and a third injection 10 days after surgery. Mice were killed 15 days after the osteotomy procedure. Bone repair was assessed using microcomputed tomography (micro-CT) and histologic evaluation of the fracture callus. Muscle healing was assessed using Masson trichrome staining of the injury site, and image analysis was used to quantify the degree of fibrosis and muscle regeneration.
Results: Three propeptide injections over a period of 15 days increased body mass by 7% and increased muscle mass by almost 20% (p < 0.001). Micro-CT analysis of the osteotomy site shows that by 15 days postosteotomy, bony callus tissue was observed bridging the osteotomy gap in 80% of the propeptide-treated mice but only 40% of the control (vehicle)-treated mice (p < 0.01). Micro-CT quantification shows that bone volume of the fracture callus was increased by ∼ 30% (p < 0.05) with propeptide treatment, and the increase in bone volume was accompanied by a significant increase in cartilage area (p = 0.01). Propeptide treatment significantly decreased the fraction of fibrous tissue in the wound site and increased the fraction of muscle relative to fibrous tissue by 20% (p < 0.01).
Conclusions: Blocking myostatin signaling in the injured limb improves fracture healing and enhances muscle regeneration. These data suggest that myostatin inhibitors may be effective for improving wound repair in cases of orthopaedic trauma and extremity injury.
Figures


Similar articles
-
A myostatin inhibitor (propeptide-Fc) increases muscle mass and muscle fiber size in aged mice but does not increase bone density or bone strength.Exp Gerontol. 2013 Sep;48(9):898-904. doi: 10.1016/j.exger.2013.06.004. Epub 2013 Jul 4. Exp Gerontol. 2013. PMID: 23832079 Free PMC article.
-
Immunolocalization of myostatin (GDF-8) following musculoskeletal injury and the effects of exogenous myostatin on muscle and bone healing.J Histochem Cytochem. 2012 Jan;60(1):22-30. doi: 10.1369/0022155411425389. J Histochem Cytochem. 2012. PMID: 22205678 Free PMC article.
-
Myostatin (GDF-8) deficiency increases fracture callus size, Sox-5 expression, and callus bone volume.Bone. 2009 Jan;44(1):17-23. doi: 10.1016/j.bone.2008.08.126. Epub 2008 Sep 13. Bone. 2009. PMID: 18852073 Free PMC article.
-
Myostatin (GDF-8) as a key factor linking muscle mass and bone structure.J Musculoskelet Neuronal Interact. 2010 Mar;10(1):56-63. J Musculoskelet Neuronal Interact. 2010. PMID: 20190380 Free PMC article. Review.
-
Pleiotropic actions of Vitamin D in composite musculoskeletal trauma.Injury. 2020 Oct;51(10):2099-2109. doi: 10.1016/j.injury.2020.06.023. Epub 2020 Jun 18. Injury. 2020. PMID: 32624209 Review.
Cited by
-
Impaired skeletal muscle regeneration in the absence of fibrosis during hibernation in 13-lined ground squirrels.PLoS One. 2012;7(11):e48884. doi: 10.1371/journal.pone.0048884. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23155423 Free PMC article.
-
Postsurgical Acute Phase Reaction is Associated with Decreased Levels of Circulating Myostatin.Inflammation. 2015 Aug;38(4):1727-30. doi: 10.1007/s10753-015-0149-6. Inflammation. 2015. PMID: 25749570
-
How Can Promoting Skeletal Muscle Health and Exercise in Children and Adolescents Prevent Insulin Resistance and Type 2 Diabetes?Life (Basel). 2024 Sep 21;14(9):1198. doi: 10.3390/life14091198. Life (Basel). 2024. PMID: 39337980 Free PMC article. Review.
-
Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations.Cold Spring Harb Perspect Med. 2017 Nov 1;7(11):a029793. doi: 10.1101/cshperspect.a029793. Cold Spring Harb Perspect Med. 2017. PMID: 28389517 Free PMC article. Review.
-
A myostatin inhibitor (propeptide-Fc) increases muscle mass and muscle fiber size in aged mice but does not increase bone density or bone strength.Exp Gerontol. 2013 Sep;48(9):898-904. doi: 10.1016/j.exger.2013.06.004. Epub 2013 Jul 4. Exp Gerontol. 2013. PMID: 23832079 Free PMC article.
References
-
- Gerstenfeld L, Einhorn T. Developmental aspects of fracture healing and the use of pharmacological agents to alter healing. J Musculoskeletal Neuronal Interact. 2003;3:297–303. - PubMed
-
- Stein A, Perren S, Cordey J, Kenwright J, Mosheiff R, Francis M. The muscle bed—a crucial factor in fracture healing: a physiological concept. Orthopedics. 2002;25:1379–1383. - PubMed
-
- Landry P, Marino A, Sadasivan K, Albright J. Effect of soft-tissue trauma on the early periosteal response of bone to injury. J Trauma. 2000;48:479–483. - PubMed
-
- Utvag S, Iversen K, Grundnes O, Reikeras O. Poor muscle coverage delays fracture healing in rats. Acta Orthop Scand. 2002;73:471–474. - PubMed
-
- Utvag S, Grundnes O, Rindal D, Reikeras O. Influence of extensive muscle injury on fracture healing in rat tibia. J Orthop Trauma. 2003;17:430–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

