Salicylic acid based small molecule inhibitor for the oncogenic Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2)
- PMID: 20170098
- PMCID: PMC2842125
- DOI: 10.1021/jm901645u
Salicylic acid based small molecule inhibitor for the oncogenic Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2)
Abstract
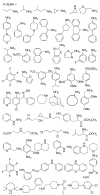
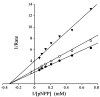
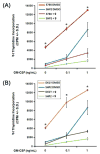
The Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2) plays a pivotal role in growth factor and cytokine signaling. Gain-of-function SHP2 mutations are associated with Noonan syndrome, various kinds of leukemias, and solid tumors. Thus, there is considerable interest in SHP2 as a potential target for anticancer and antileukemia therapy. We report a salicylic acid based combinatorial library approach aimed at binding both active site and unique nearby subpockets for enhanced affinity and selectivity. Screening of the library led to the identification of a SHP2 inhibitor II-B08 (compound 9) with highly efficacious cellular activity. Compound 9 blocks growth factor stimulated ERK1/2 activation and hematopoietic progenitor proliferation, providing supporting evidence that chemical inhibition of SHP2 may be therapeutically useful for anticancer and antileukemia treatment. X-ray crystallographic analysis of the structure of SHP2 in complex with 9 reveals molecular determinants that can be exploited for the acquisition of more potent and selective SHP2 inhibitors.
Figures
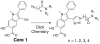

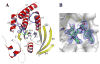
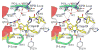
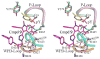


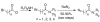
Similar articles
-
Targeting protein tyrosine phosphatase SHP2 for the treatment of PTPN11-associated malignancies.Mol Cancer Ther. 2013 Sep;12(9):1738-48. doi: 10.1158/1535-7163.MCT-13-0049-T. Epub 2013 Jul 3. Mol Cancer Ther. 2013. PMID: 23825065 Free PMC article.
-
Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases.Nature. 2016 Jul 7;535(7610):148-52. doi: 10.1038/nature18621. Epub 2016 Jun 29. Nature. 2016. PMID: 27362227
-
Therapeutic potential of targeting the oncogenic SHP2 phosphatase.J Med Chem. 2014 Aug 14;57(15):6594-609. doi: 10.1021/jm5006176. Epub 2014 Jul 28. J Med Chem. 2014. PMID: 25003231 Free PMC article.
-
Recent Advances of SHP2 Inhibitors in Cancer Therapy: Current Development and Clinical Application.J Med Chem. 2020 Oct 22;63(20):11368-11396. doi: 10.1021/acs.jmedchem.0c00249. Epub 2020 Jun 10. J Med Chem. 2020. PMID: 32460492 Review.
-
The Landscape of Protein Tyrosine Phosphatase (Shp2) and Cancer.Curr Pharm Des. 2018;24(32):3767-3777. doi: 10.2174/1381612824666181106100837. Curr Pharm Des. 2018. PMID: 30398108 Review.
Cited by
-
Bicyclic benzofuran and indole-based salicylic acids as protein tyrosine phosphatase inhibitors.Bioorg Med Chem. 2012 Mar 15;20(6):1940-6. doi: 10.1016/j.bmc.2011.11.004. Epub 2011 Nov 9. Bioorg Med Chem. 2012. PMID: 22133902 Free PMC article.
-
Targeting SHP2 phosphatase in myeloproliferative neoplasms.Oncotarget. 2012 Oct;3(10):1049-51. doi: 10.18632/oncotarget.665. Oncotarget. 2012. PMID: 23165317 Free PMC article. No abstract available.
-
SHP2 is a target of the immunosuppressant tautomycetin.Chem Biol. 2011 Jan 28;18(1):101-10. doi: 10.1016/j.chembiol.2010.10.015. Chem Biol. 2011. PMID: 21276943 Free PMC article.
-
The protein tyrosine phosphatase, Shp2, positively contributes to FLT3-ITD-induced hematopoietic progenitor hyperproliferation and malignant disease in vivo.Leukemia. 2013 Feb;27(2):398-408. doi: 10.1038/leu.2012.308. Epub 2012 Oct 22. Leukemia. 2013. PMID: 23103841 Free PMC article.
-
Therapeutic potential of targeting protein tyrosine phosphatases in liver diseases.Acta Pharm Sin B. 2024 Aug;14(8):3295-3311. doi: 10.1016/j.apsb.2024.05.006. Epub 2024 May 13. Acta Pharm Sin B. 2024. PMID: 39220870 Free PMC article. Review.
References
-
- Tonks NK, Neel BG. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr Opin Cell Biol. 2001;13:182–195. - PubMed
-
- Zhang ZY. Protein tyrosine phosphatases: prospects for therapeutics. Curr Opin Chem Biol. 2001;5:416–423. - PubMed
-
- Ventura JJ, Nebreda AR. Protein kinases and phosphatases as therapeutic targets in cancer. Clin Transl Oncol. 2006;8:153–160. - PubMed
-
- Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–187. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

