Comparison on functional assays for Gq-coupled GPCRs by measuring inositol monophospate-1 and intracellular calcium in 1536-well plate format
- PMID: 20161830
- PMCID: PMC2774619
- DOI: 10.2174/1875397300801010070
Comparison on functional assays for Gq-coupled GPCRs by measuring inositol monophospate-1 and intracellular calcium in 1536-well plate format
Abstract
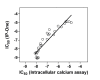
Cell-based functional assays used for compound screening and lead optimization play an important role in drug discovery for G-protein coupled receptors (GPCRs). Cell-based assays can define the role of a compound as an agonist, antagonist or inverse agonist and can provide detailed information about the potency and efficacy of a compound. In addition, cell-based screens can be used to identify allosteric modulators that interact with sites other than the binding site of the endogenous ligand. Intracellular calcium assays which use a fluorescent calcium binding dye (such as Fluo-3, Fluo-4 or Fura-2) have been used in compound screening campaigns to measure the activity of Gq-coupled GPCRs. However, such screening methodologies require a special instrumentation to record the rapid change in intracellular free calcium concentration over time. The radioactive inositol 1,4,5- triphosphate (IP(3)) assay measures (3)H-inositol incorporation and is another traditional assay for the assessment of Gq-coupled GPCR activity, but it is not suitable for screening of large size compound collections because it requires a cell wash step and generates radioactive waste. To avoid these limitations, we have optimized and miniaturized a TR-FRET based IP-One assay that measures inositol monophosphate in a 1536-well plate format. This assay is homogenous, non-radioactive and does not require a kinetic readout. It has been tested with the cell lines expressing M(1) acetylcholine, FFAR1, vasopressin V1b, or Neuropeptide S receptors. The activities of antagonists determined in the IP-One assay correlated well with these measured in the intracellular calcium assay while the correlation of agonist activities might vary from cell line to cell line. This IP-One assay offers an alternative method for high throughput screening of Gq-coupled GPCRs without using costly kinetic plate readers.
Figures





Similar articles
-
Assessing Gαq/15-signaling with IP-One: Single Plate Transfection and Assay Protocol for Cell-Based High-Throughput Assay.Bio Protoc. 2020 Aug 20;10(16):e3715. doi: 10.21769/BioProtoc.3715. eCollection 2020 Aug 20. Bio Protoc. 2020. PMID: 33659379 Free PMC article.
-
Miniaturization of intracellular calcium functional assays to 1536-well plate format using a fluorometric imaging plate reader.J Biomol Screen. 2004 Aug;9(5):417-26. doi: 10.1177/1087057104264038. J Biomol Screen. 2004. PMID: 15296641
-
Monitoring Gq-coupled receptor response through inositol phosphate quantification with the IP-One assay.Expert Opin Drug Discov. 2011 Oct;6(10):981-94. doi: 10.1517/17460441.2011.608658. Epub 2011 Sep 8. Expert Opin Drug Discov. 2011. PMID: 22646860
-
An overview of Ca2+ mobilization assays in GPCR drug discovery.Expert Opin Drug Discov. 2017 May;12(5):511-523. doi: 10.1080/17460441.2017.1303473. Epub 2017 Mar 17. Expert Opin Drug Discov. 2017. PMID: 28277837 Review.
-
Emerging concepts of guanine nucleotide-binding protein-coupled receptor (GPCR) function and implications for high throughput screening.Assay Drug Dev Technol. 2007 Jun;5(3):425-51. doi: 10.1089/adt.2007.062. Assay Drug Dev Technol. 2007. PMID: 17638542 Review.
Cited by
-
Potentiation of sulfonylurea action by an EPAC-selective cAMP analog in INS-1 cells: comparison of tolbutamide and gliclazide and a potential role for EPAC activation of a 2-APB-sensitive Ca2+ influx.Mol Pharmacol. 2013 Jan;83(1):191-205. doi: 10.1124/mol.112.081943. Epub 2012 Oct 15. Mol Pharmacol. 2013. PMID: 23071106 Free PMC article.
-
The novel peptide LCGM-10 attenuates metabotropic glutamate receptor 5 activity and demonstrates behavioral effects in animal models.Front Behav Neurosci. 2024 Feb 7;18:1333258. doi: 10.3389/fnbeh.2024.1333258. eCollection 2024. Front Behav Neurosci. 2024. PMID: 38385004 Free PMC article.
-
G protein-coupled receptor signaling analysis using homogenous time-resolved Förster resonance energy transfer (HTRF®) technology.Int J Mol Sci. 2014 Feb 13;15(2):2554-72. doi: 10.3390/ijms15022554. Int J Mol Sci. 2014. PMID: 24531140 Free PMC article.
-
A Multiplexed Fluorescent Calcium and NFAT Reporter Gene Assay to Identify GPCR Agonists.Curr Chem Genom Transl Med. 2013 Apr 3;7:1-8. doi: 10.2174/2213988501307010001. eCollection 2013. Curr Chem Genom Transl Med. 2013. PMID: 24396729 Free PMC article.
-
Novel Agonist Bioisosteres and Common Structure-Activity Relationships for The Orphan G Protein-Coupled Receptor GPR139.Sci Rep. 2016 Nov 10;6:36681. doi: 10.1038/srep36681. Sci Rep. 2016. PMID: 27830715 Free PMC article.
References
-
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727–30. - PubMed
-
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–50. - PubMed
-
- Eglen RM, Bosse R, Reisine T. Emerging concepts of guanine nucleotide-binding protein-coupled receptor (GPCR) function and implications for high throughput screening. Assay Drug Dev Technol. 2007;5(3):425–51. - PubMed
-
- Inglese J, Johnson RL, Simeonov A, Xia M, Zheng W, Austin CP, Auld DS. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 2007;3(8):466–79. - PubMed
-
- Prystay L, Gagne A, Kasila P, Yeh LA, Banks P. Homogeneous cell-based fluorescence polarization assay for the direct detection of cAMP. J Biomol Screen. 2001;6(2):75–82. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
