A Bax-mediated mechanism for obatoclax-induced apoptosis of cholangiocarcinoma cells
- PMID: 20160031
- PMCID: PMC2831099
- DOI: 10.1158/0008-5472.CAN-09-3535
A Bax-mediated mechanism for obatoclax-induced apoptosis of cholangiocarcinoma cells
Abstract
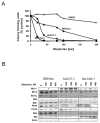
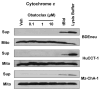
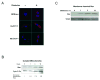
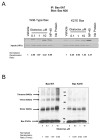
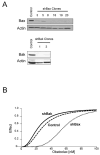
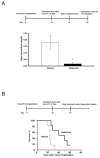
Apoptosis induction by BH3 mimetics is a therapeutic strategy for human cancer. These mimetics exert single-agent activity in cells "primed" for cell death. Primed cells are dependent upon antiapoptotic Bcl-2 proteins for survival and are characterized by the ability of the BH3 mimetic to induce cytochrome c release from their isolated mitochondria. Our aim was to examine the single-agent activity of obatoclax, a BH3 mimetic in cholangiocarcinoma cell lines. In clonogenic assays, inhibition of colony formation was observed by obatoclax treatment. Despite single-agent activity by obatoclax, the mitochondria from these cells did not release cytochrome c after incubation with this BH3 mimetic. However, immunofluorescence and cell fractionation studies identified Bax activation and translocation to mitochondria after treatment with obatoclax. shRNA targeted knockdown of Bax doubled the IC50 for obatoclax but did not abrogate its cytotoxicity, whereas knockdown of Bak did not alter the IC50. In a cell-free system, obatoclax induced an activating conformational change of Bax, which was attenuated by a site-directed mutagenesis of a previously identified protein activation site. Finally, the drug also elicited a significant in vivo response in a rodent model of this disease. In conclusion, single-agent obatoclax treatment results in Bax activation, which contributes, in part, to cell death in cholangiocarcinoma cells. These data indicate that BH3 mimetics may also function as direct activators of Bax and induce cytotoxicity in cells not otherwise primed for cell death.
Figures
Similar articles
-
BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells.Clin Cancer Res. 2009 Jan 1;15(1):150-9. doi: 10.1158/1078-0432.CCR-08-1575. Clin Cancer Res. 2009. PMID: 19118042 Free PMC article.
-
BH3-only protein mimetic obatoclax sensitizes cholangiocarcinoma cells to Apo2L/TRAIL-induced apoptosis.Mol Cancer Ther. 2008 Aug;7(8):2339-47. doi: 10.1158/1535-7163.MCT-08-0285. Mol Cancer Ther. 2008. PMID: 18723481 Free PMC article.
-
Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax).Cancer Res. 2008 May 1;68(9):3413-20. doi: 10.1158/0008-5472.CAN-07-1919. Cancer Res. 2008. PMID: 18451169 Free PMC article.
-
Human melanoma cells under endoplasmic reticulum stress are more susceptible to apoptosis induced by the BH3 mimetic obatoclax.Neoplasia. 2009 Sep;11(9):945-55. doi: 10.1593/neo.09692. Neoplasia. 2009. PMID: 19724688 Free PMC article.
-
Autophagy contributes to modulating the cytotoxicities of Bcl-2 homology domain-3 mimetics.Semin Cancer Biol. 2013 Dec;23(6 Pt B):553-60. doi: 10.1016/j.semcancer.2013.08.008. Epub 2013 Sep 4. Semin Cancer Biol. 2013. PMID: 24012660 Review.
Cited by
-
HDAC6 inhibition restores ciliary expression and decreases tumor growth.Cancer Res. 2013 Apr 1;73(7):2259-70. doi: 10.1158/0008-5472.CAN-12-2938. Epub 2013 Jan 31. Cancer Res. 2013. PMID: 23370327 Free PMC article.
-
B Cell Lymphoma 2: A Potential Therapeutic Target for Cancer Therapy.Int J Mol Sci. 2021 Sep 28;22(19):10442. doi: 10.3390/ijms221910442. Int J Mol Sci. 2021. PMID: 34638779 Free PMC article. Review.
-
Mcl-1 and YY1 inhibition and induction of DR5 by the BH3-mimetic Obatoclax (GX15-070) contribute in the sensitization of B-NHL cells to TRAIL apoptosis.Cell Cycle. 2011 Aug 15;10(16):2792-805. doi: 10.4161/cc.10.16.16952. Epub 2011 Aug 15. Cell Cycle. 2011. PMID: 21822052 Free PMC article.
-
Comparison of in vitro antileukemic activity of obatoclax and ABT-737.Tumour Biol. 2016 Aug;37(8):10839-49. doi: 10.1007/s13277-016-4943-z. Epub 2016 Feb 15. Tumour Biol. 2016. PMID: 26880588 Free PMC article.
-
Targeting the Bcl-2 family for cancer therapy.Expert Opin Ther Targets. 2013 Jan;17(1):61-75. doi: 10.1517/14728222.2013.733001. Epub 2012 Nov 22. Expert Opin Ther Targets. 2013. PMID: 23173842 Free PMC article. Review.
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. - PubMed
-
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature Reviews Molecular Cell Biology. 2008;9(1):47–59. - PubMed
-
- Jabbour AM, Heraud JE, Daunt CP, et al. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death & Differentiation. 2009;16(4):555–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

